PEM Electrolyzer, Paper Numerical Validation, ANSYS Fluent
$1,170.00 $468.00 HPC
- This project numerically simulates the PEM Fuel Cell using ANSYS Fluent software.
- We design the 3-D model with the Design Modeler software.
- We mesh the model with ANSYS Meshing software to generate a Structured mesh.
- We used the Potential/Electrochemistry model to define the potential equations.
- We used the Electrolysis sub-model and PEM Electrolysis type to define the electrolysis process.
- We used the Species model to define H2, O2, and H2O.
- We used the Mixture Multiphase model to define water and species phases.
- The present CFD work is Validated with a reference Article.
To Order Your Project or benefit from a CFD consultation, contact our experts via email (info@mr-cfd.com), online support tab, or WhatsApp at +44 7443 197273.
There are some Free Products to check our service quality.
If you want the training video in another language instead of English, ask it via info@mr-cfd.com after you buy the product.
Description
Description
In this project, we performed a CFD simulation of a PEM Electrolyzer via ANSYS Fluent software.
This product is provided according to the simulation of a reference article, “3D CFD analysis of species transport and electrochemical behavior in a PEM electrolyzer”. Then, the results of the present work are compared and validated with the reference article.
A Fuel Cell is an energy conversion device that converts chemical energy into electrical energy. In contrast, an Electrolyzer operates in the reverse of a fuel cell system. It means power (electricity) is consumed to produce fuel (hydrogen).
Similar to fuel cells, electrolyzers consist of two main sides called anode and cathode, which are connected by an electrolyte membrane layer. Each anode or cathode side includes a catalyst layer, a current collector, a gas diffusion layer (porous electrode), and a gas flow channel.
Electrochemical reactions take place in catalyst layers on the anode and cathode sides. As a result of these reactions, incoming water is consumed to produce oxygen at the anode and hydrogen at the cathode.
Methodology
We performed the simulation process via ANSYS Fluent software. First, we modeled the geometry in 3D using Design Modeler software. Next, we meshed the model using ANYS Meshing software. The grid type is structured, and 94,800 cells are generated.
In the simulation procedure, we used the Potential/Electrochemistry model to define the potential equation. Then, we utilized the Electrolysis sub-model to define the electrolysis system. As a type of electrolysis process, we selected the PEM Electrolysis (proton-exchange membrane electrolysis).
When we use the PEM electrolysis model, the Multiphase model and Species model are automatically enabled. According to the Mixture Multiphase model, water is defined as a secondary phase, and a species combination is defined as a primary phase. For the primary phase, the Species Transport model defines the species participating in the electrolysis mechanism, including hydrogen and oxygen.
Conclusion
For post-processing, we obtained contours corresponding to the distributions of H2, O2, and H2O. So, we provided the volume fraction of water and the mass fraction of oxygen and hydrogen in the middle of the electrolyzer at different potentials.
The contours of H2 and O2 mass fraction show that oxygen is generated at the anode side, and hydrogen is generated at the cathode side, due to chemical reactions. This behavior confirms that the present PEM electrolyzer system operates correctly.
The results show that as the cell voltage increases, the volume fraction of consumed water decreases and the mass fraction of produced hydrogen and oxygen increases. This is because when the cell potential field increases, the electrochemical reactions in the electrolyzer system are augmented.
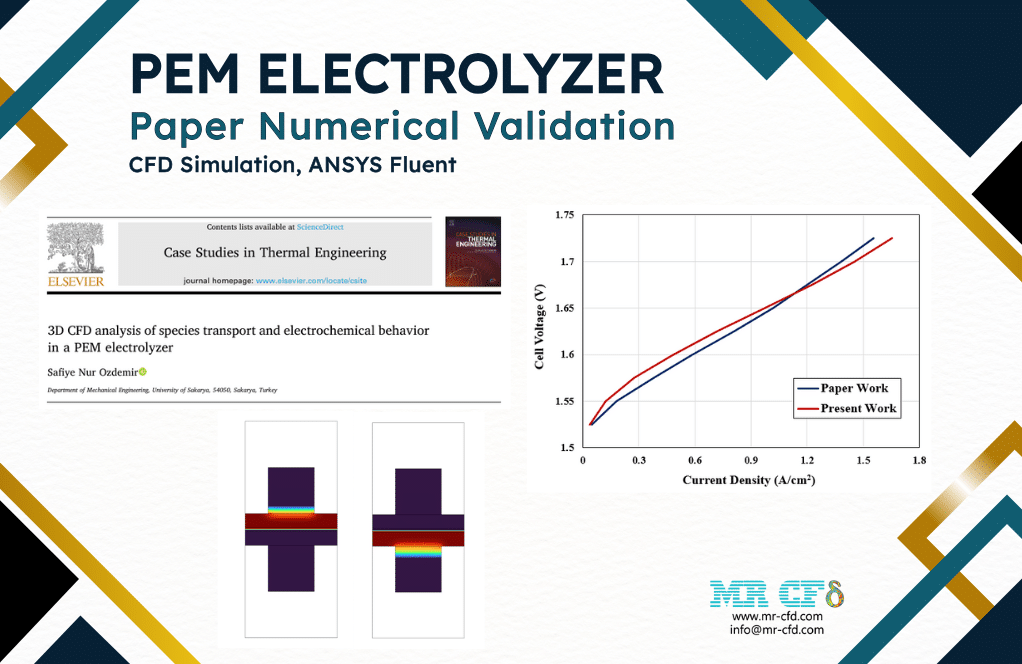
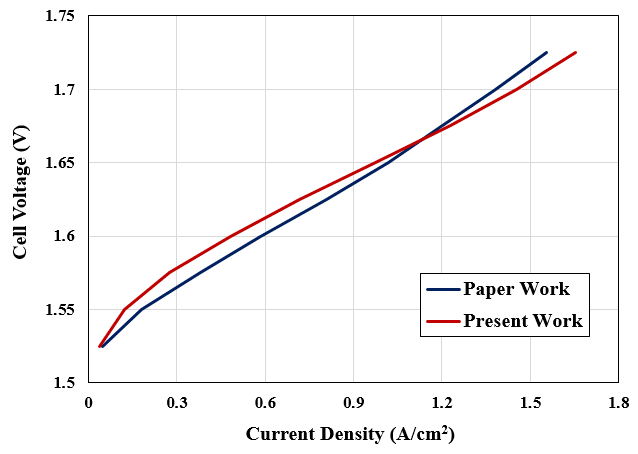
We performed a validation procedure for our present numerical study based on one of the Polarization Curves mentioned in the reference article. The polarization curve presents the Current Density variation with respect to the Cell Voltage in the PEM electrolyzer system.
Therefore, we implemented the present simulation project in several cases. In each case study, we defined different voltages or potentials (1.525 to 1.725 volts) and then computed the current density for each case.
So, we presented the results in the following curve, showing that the present numerical work is highly accurate and exact.
You must be logged in to post a review.



Reviews
There are no reviews yet.