Project Outsourcing
Outsource your project to the MR CFD simulation engineering team. Our experts are ready to carry out every CFD project in all related engineering fields. Our services include industrial and academic purposes, considering the ANSYS Fluent software's wide range of CFD simulations. By outsourcing your project, you can benefit from MR CFD's primary services, including Consultation, Training, and CFD Simulation. The project freelancing procedure is as follows:
An official contract will be set based on your project description and details.
As we start your project, you will have access to our Portal to track its progress.
You will receive the project's resource files after you confirm the final report.
Finally, you will receive a comprehensive training video and technical support.
What are Fuel Cells?
Fuel cells are devices that directly convert chemical energy to electricity through electrochemical reactions. Although there is a resemblance between fuel cells and batteries, their specific characteristics make them widely used in industries and be under the scrutiny of many researchers. Indeed, they can be used as the primary or backup power supply in hospitals, hotels, public transportation, automobiles and etc.
Moreover, they are constructed from electrodes, one as a cathode and another as an anode, catalysts, electrolytes, fuel, and an oxidizer. To describe in brief, a hydrogen-rich fuel like natural gas enters the cell and reacts with oxygen (ambient air) electrochemically to generate electricity, heat, and water. The reactions occur at electrodes and the electrolyte is responsible for carrying electrically charged particles from one electrode to another. Plus, the catalyst could boost the reactions up at electrodes.
Fuel cells are classified based on different parameters, the most important of which is the material they employ. This would completely change its operating temperature range, power output, potential application, limitations, etc. Thus, there are different classifications, but for now, four major types of them, including Alkaline, Molten carbonate, Phosphoric acid & Solid oxide, are summarized in the below table:
| Type of Fuel Cell | Electrolyte | Anode & Cathode | Power Output (Watt) | Efficiency | Weak Points | Description |
| Alkaline (AFC) | Solution of Potassium Hydroxide – Sodium Hydroxide in water | Hydrogen – Oxygen | 300 ~ 5000 | 40~ 70% | Risk of Leaking-
Poisoning by CO2- Needs Pure Hydrogen fuel- Expensive Platinum catalyst |
Operating Temp = 150 ~ 200 C
– Used in Spacecraft Industry |
| Molten Carbonate (MCFC) | Salt Carbonate – Molten Potassium Lithium Carbonate | Hydrogen – Oxygen & Carbon Dioxide | 2MW ~ 10^2MW | 60 ~ 80% | Limited Life cycle due to high temperature – cannot use at-home application- need CO2 | Operating Temp = 650 C
– Nickel Electrode is inexpensive – Used in industrial & Military application |
| Phosphoric Acid
(PAFC) |
Phosphoric acid | Hydrogen – Oxygen | 200KW ~ 11MW | 40 ~ 80% | Expensive platinum catalyst- Big size and weight | Operating Temp = 150 ~ 200 C
– Tolerate different fuels sources |
| Solid Oxide (SOFC) | Compound metal oxides like Calcium or Zirconium | Hydrogen – Oxygen | 100KW | 60% | Slow startup –
Limited potential application- Big size- capable of cracking |
Operating Temp = 1000 C –
Sulfur-resistant – doesn`t need a reformer |
| Proton Exchange Membrane (PEMFC) | Polymer | Hydrogen – Oxygen | 50~250KW | 40~50% | Expensive platinum catalyst – Pure fuel required | Operating Temp = 80 C- viable for home & vehicles application |
Fuel Cell Simulation via ANSYS Fluent
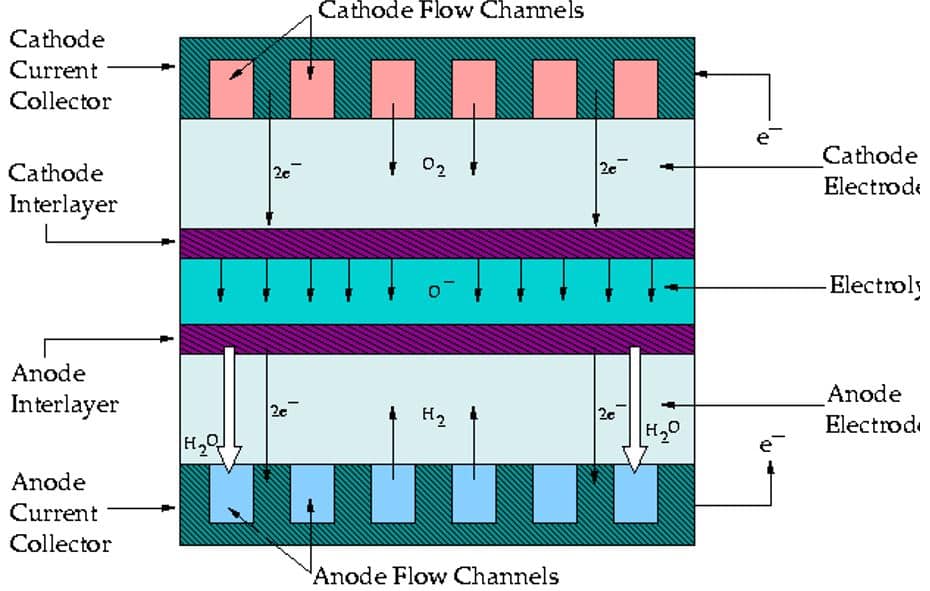
ANSYS Fluent provides fuel cells and electrolysis as an add-on module. For this module, you need to define electrochemical parameters related to anode & cathode, current collector zone, flow channel, porous electrolyte, and catalyst layer for both anode & cathode and also electrolyte zone (figure 1).
Note that there are more detailed settings if there is any coolant channel or contact resistance. Contact Resistivity is used to deal with some of the cell contact surfaces, a jump in the electrical potential caused by incomplete conduction. The Coolant Channel mode is used for cases where the cooling channel exists in the fuel cell, and the Stack Management mode is used when multiple fuel cell units are combined with increasing efficiency.
Current collectors are solid materials with thermal energy sources and electrical potential. The flow channels carry a mixture of gaseous species, including oxygen, hydrogen, and water. The catalytic part consists of a porous medium and contains mass sources, thermal energy, electrical potential, proton potential, saturated water, hydrogen, oxygen, and water. The gaseous diffusion zone comprises a porous medium and contains mass sources, thermal energy, electrical potential, saturated water, and hydrogen, oxygen, and gaseous water species.
The polymer membrane region also comprises a porous medium with thermal energy sources and proton potentials. All in all, the simulation process may seem to be confusing at first sight, but you can count on MR-CFD products, which could be very helpful through learning. Here are a fuel cell product related to the PEMFC model:

You may find the related products to the Fuel Cell simulation category in Training Shop.
Our services are not limited to the mentioned subjects, and the MR-CFD is ready to undertake different and challenging projects in the Fuel Cell Engineering modeling field ordered by our customers. We even accept carrying out CFD simulation for any abstract or concept design you have in your mind to turn them into reality and even help you reach the best design for what you may have imagined. You can benefit from MR-CFD expert consultation for free, and then order your project to be simulated and trained.
By outsourcing your project to the MR-CFD as a CFD simulation freelancer, you will not only receive the related project’s resource files (Geometry, Mesh, Case & Data, …), but also you will be provided with an extensive tutorial video demonstrating how you can create the geometry, mesh, and define the needed settings(pre-processing, processing and post-processing) in the ANSYS Fluent software all by yourself. Additionally, post-technical support is available to clarify issues and ambiguities.