Combustion in the Presence of EHD, CFD Simulation
$270.00 Student Discount
The present simulation is about combustion in the presence of EHD via ANSYS Fluent, and the results of this simulation have been analyzed.
Click on Add To Cart and obtain the Geometry file, Mesh file, and a Comprehensive ANSYS Fluent Training Video.To Order Your Project or benefit from a CFD consultation, contact our experts via email ([email protected]), online support tab, or WhatsApp at +44 7443 197273.
There are some Free Products to check our service quality.
If you want the training video in another language instead of English, ask it via [email protected] after you buy the product.
Description
Combustion in the Presence of EHD, CFD Simulation ANSYS Fluent Training
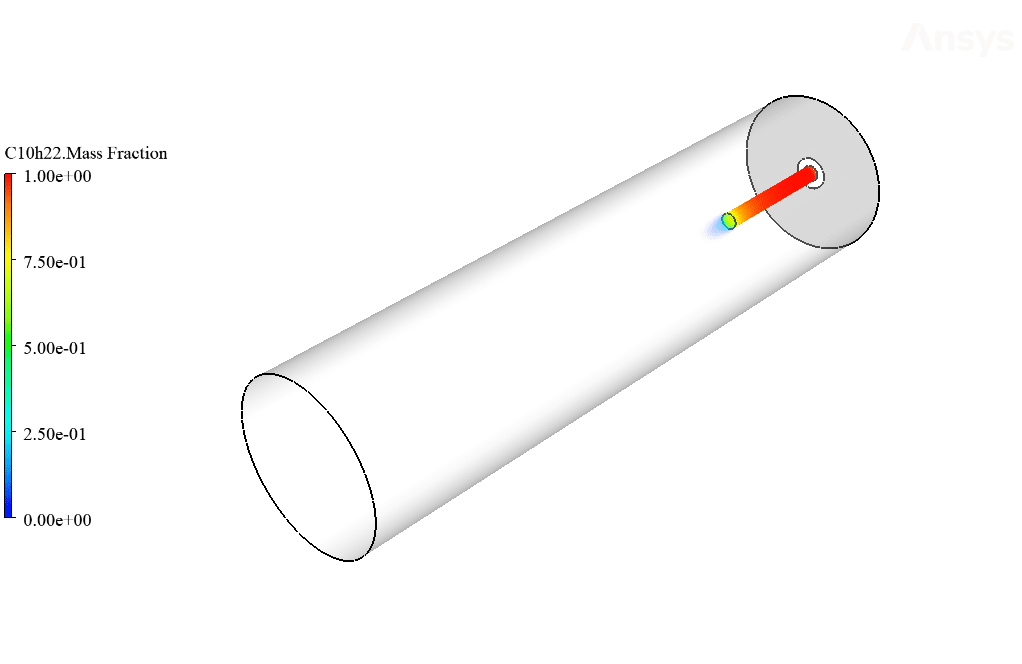
The present simulation is about combustion in the presence of EHD via ANSYS Fluent. In this project, a simple combustion chamber is designed. Airflow and fuel enter the cylindrical combustion chamber axially.
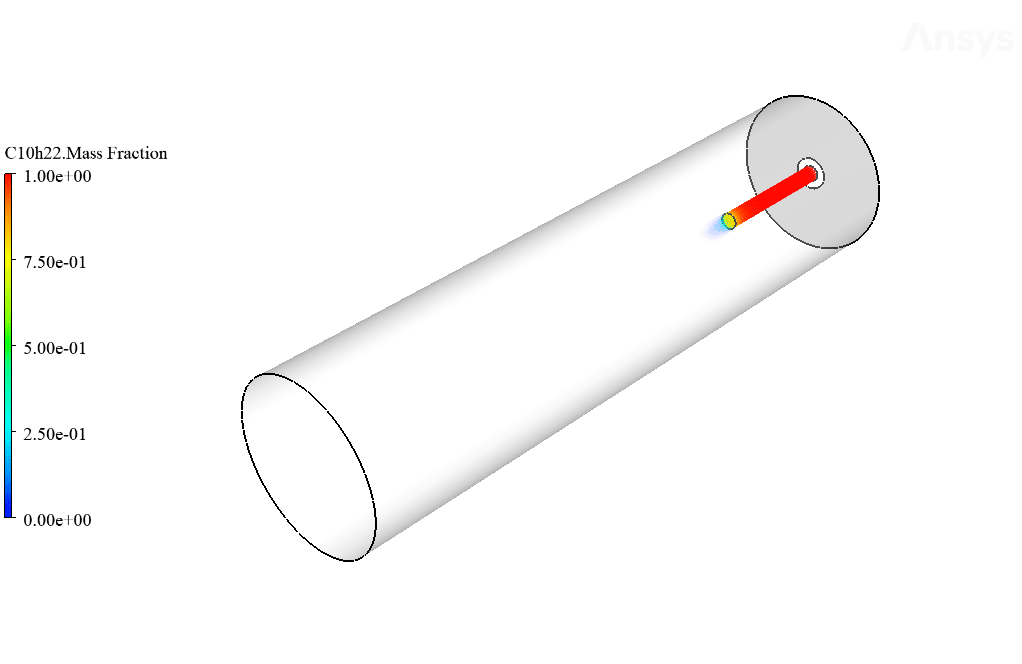
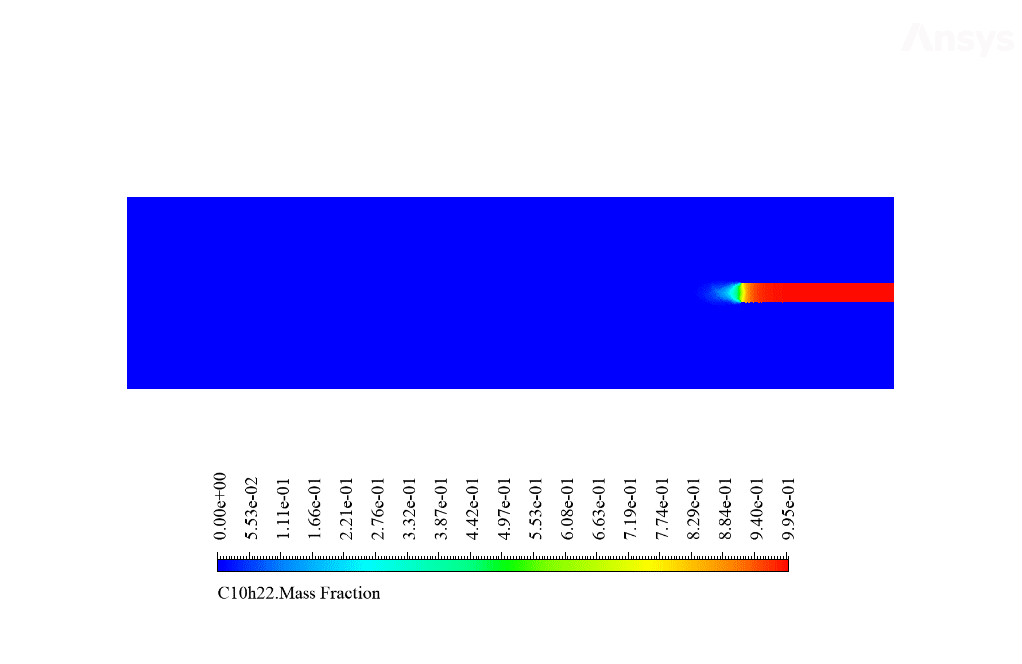
So that C10H22 enters the chamber as fuel from the central part and airflow around it. This project has been done in two steps. First, simple combustion between air and fuel is investigated. Then the same combustion is performed in the presence of an electrohydrodynamic (EHD).
Applying EHD to the fluid causes the fluid to become charged. The motion of ionized particles or molecules and their interaction with the electric field and surrounding fluid are studied. To define the combustion reaction, the Species Transport model must be used.
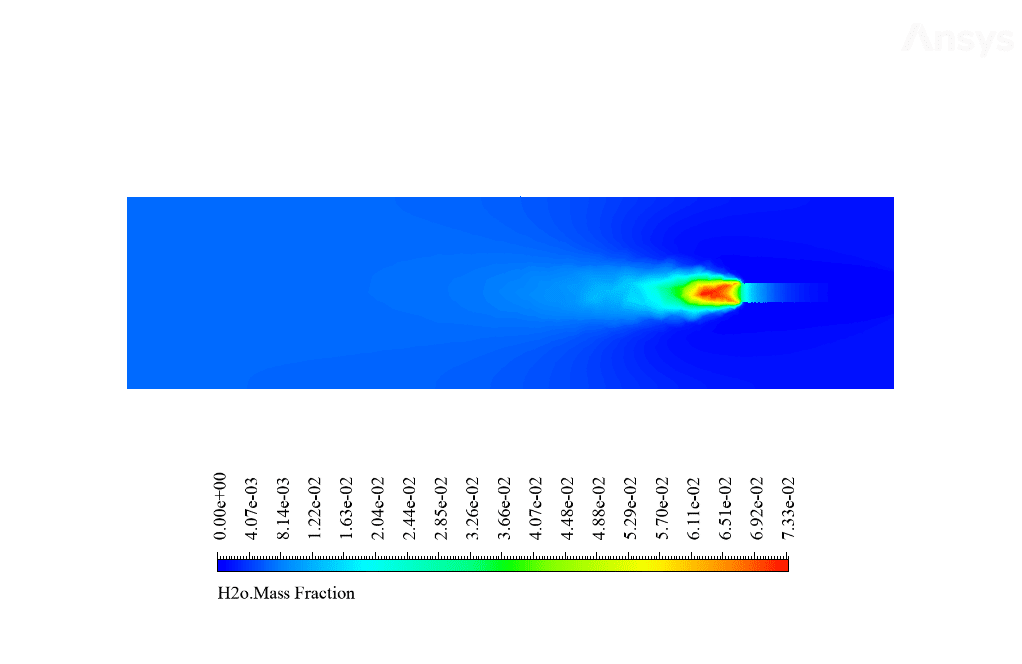
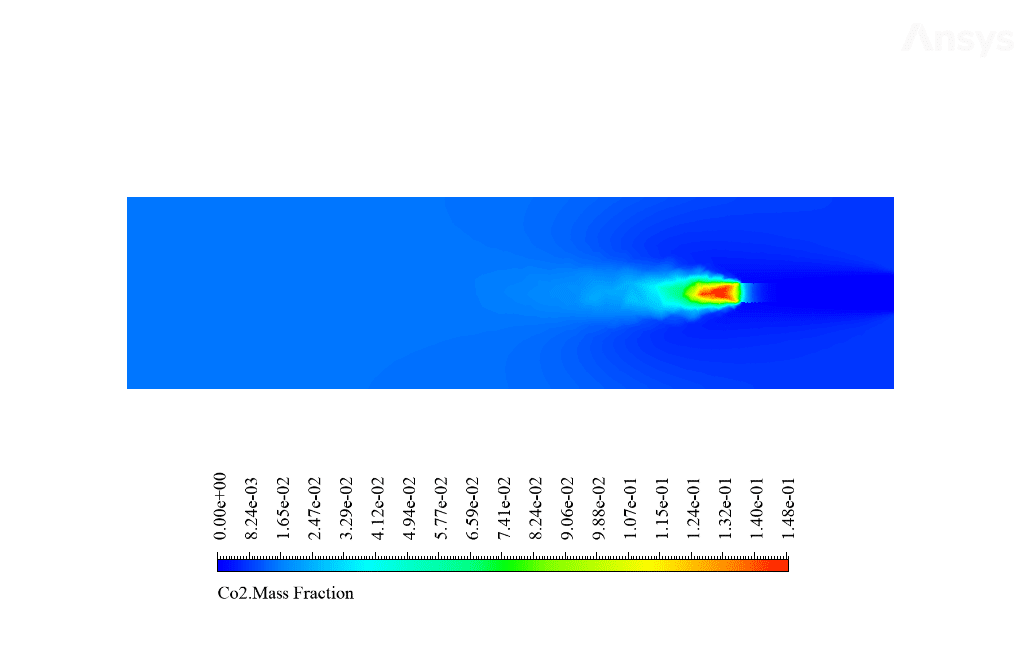
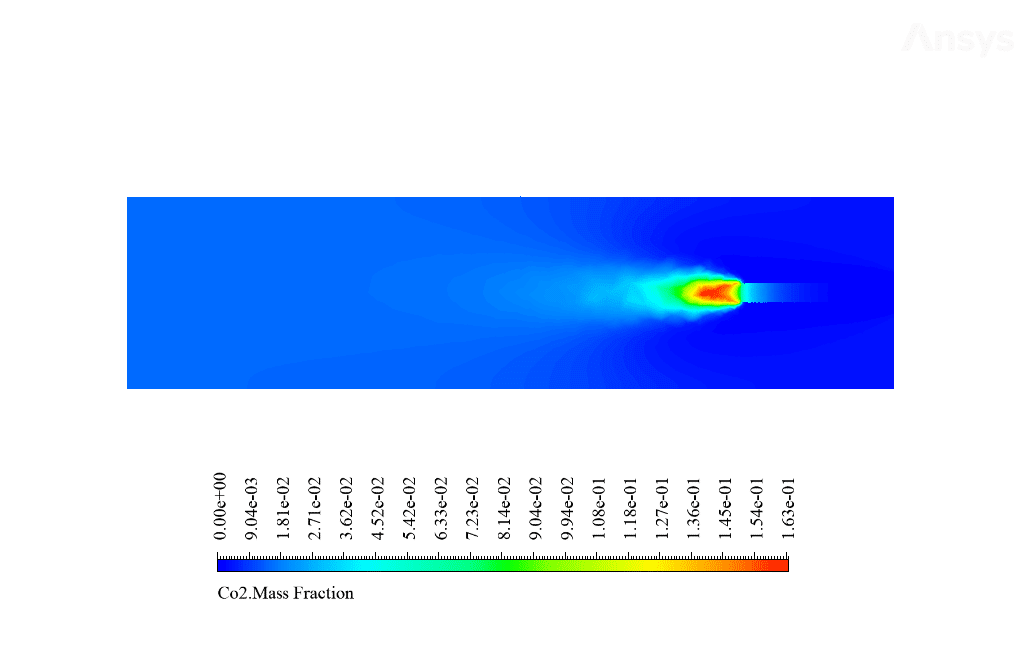
C10H22 and O2 are defined as reactants, CO2 and H2O as products in this reaction, C10H22, and O2 are defined as reactants, and CO2 and H2O are as products. Airflow with a temperature of 447 K and a velocity of 5 m / s and fuel with a temperature of 300 K and a velocity of 0.01 m / s enter the combustion chamber.
The EHD model is used to apply the electric field’s effect on the combustion chamber’s performance. 40 A / m2 is applied to the inlet and outlet boundaries of the combustion chamber. The positive charge is defined on the input boundary, and the negative charge is defined on the output boundary.
Defined combustion reaction:
Geometry & Mesh
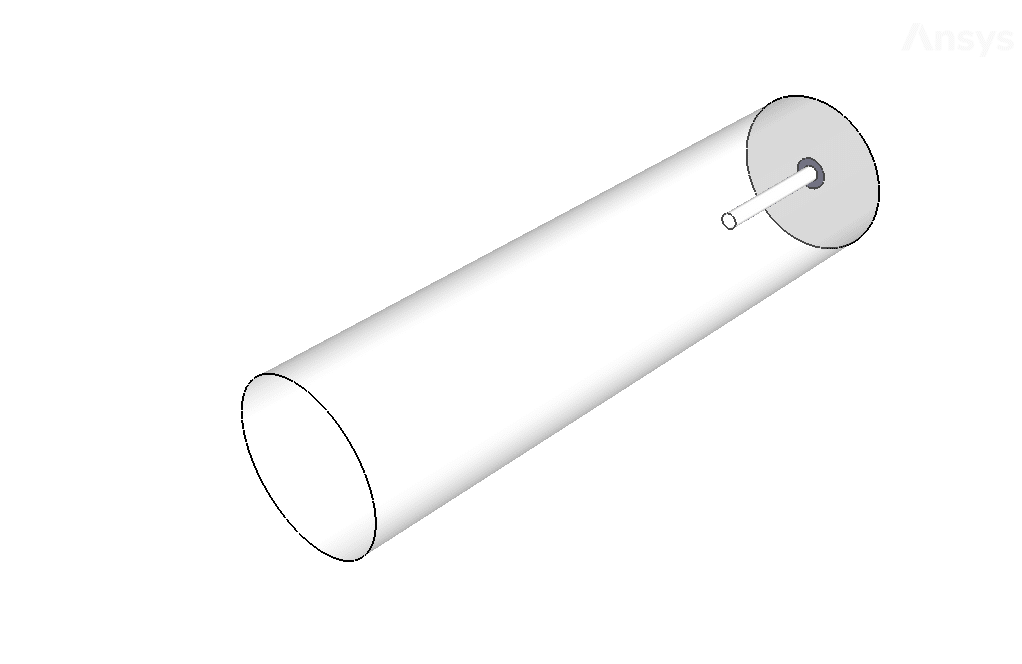
The present geometry is designed in a 3D model via Design Modeler. The computational zone of the interior is a horizontal cylindrical combustion chamber. Fuel enters the chamber through a narrow inner tube, and airflow enters the chamber from around this tube.
The mesh of the present model has been done via ANSYS Meshing. Mesh is done unstructured, and the number of production cells equals 1000658.
Set-up & Solution
Assumptions used in this simulation:
- Pressure-based solver is used.
- The present simulation is steady.
- The effect of gravity on the model is ignored.
| Models | ||
| Viscous | k-epsilon | |
| k-epsilon model | standard | |
| near wall treatment | standard wall function | |
| Species | Species Transport | |
| number of volumetric species | 5 (C10H22, O2,CO2, H2O, N2) | |
| reactions | volumetric | |
| Energy | On | |
| Potential/Li-ion Battery | On | |
| Boundary conditions | ||
| Inlet-Air | Velocity Inlet | |
| velocity magnitude | 5 m.s-1 | |
| temperature | 447 K | |
| O2 mass fraction | 0.21 | |
| C10H22, H2O, CO2 mass fraction | 0 | |
| current density | -40 A.m-2 | |
| Inlet-Fuel | Velocity Inlet | |
| velocity magnitude | 0.01 m.s-1 | |
| temperature | 300 K | |
| C10H22 mass fraction | 1 | |
| O2, H2O, CO2 mass fraction | 0 | |
| current density | 0 A.m-2 | |
| Outlet | Pressure Outlet | |
| gauge pressure | 0 Pascal | |
| current density | 40 A.m-2 | |
| Inner Wall | Wall | |
| wall motion | stationary wall | |
| thermal condition | coupled | |
| Outer Wall | Wall | |
| wall motion | stationary wall | |
| heat flux | 0 W.m-2 | |
| current density | 0 A.m-2 | |
| Methods | ||
| Pressure-Velocity Coupling | Coupled | |
| pressure | second order | |
| momentum | second order upwind | |
| turbulent kinetic energy | first order upwind | |
| turbulent dissipation rate | first order upwind | |
| species’ mass fraction | second order upwind | |
| energy | second order upwind | |
| Initialization | ||
| Initialization methods | Standard | |
| gauge pressure | 0 Pascal | |
| O2 mass fraction | 0.21 | |
| C10H22, H2O, CO2 mass fraction | 0 | |
| velocity | 5 m.s-1 | |
| temperature | 447 K | |
| Potential | 0 | |
Combustion in the Presence of EHD Results
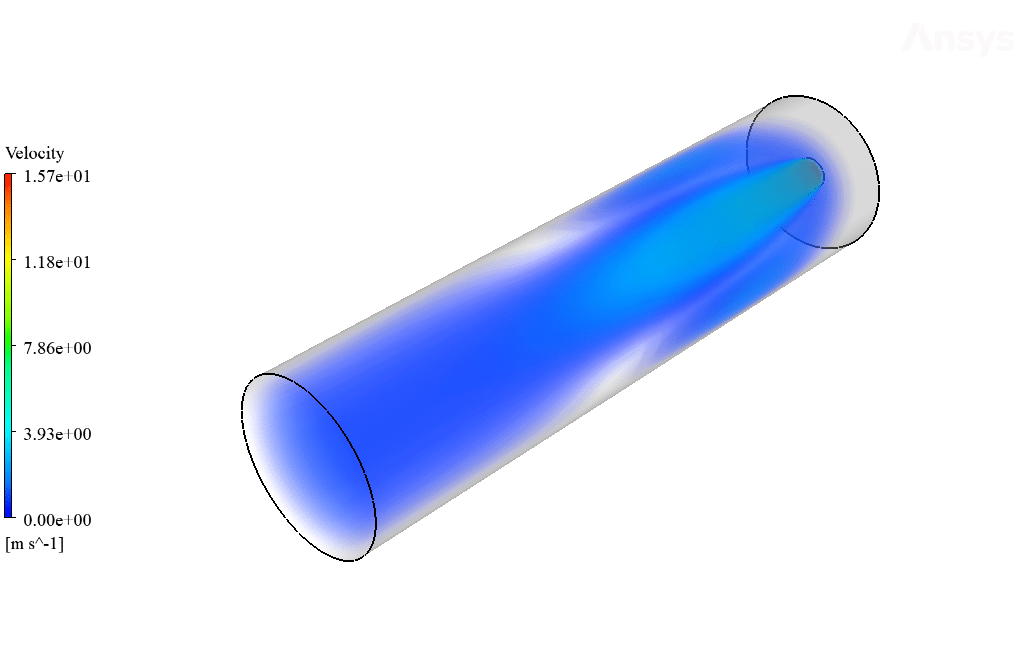
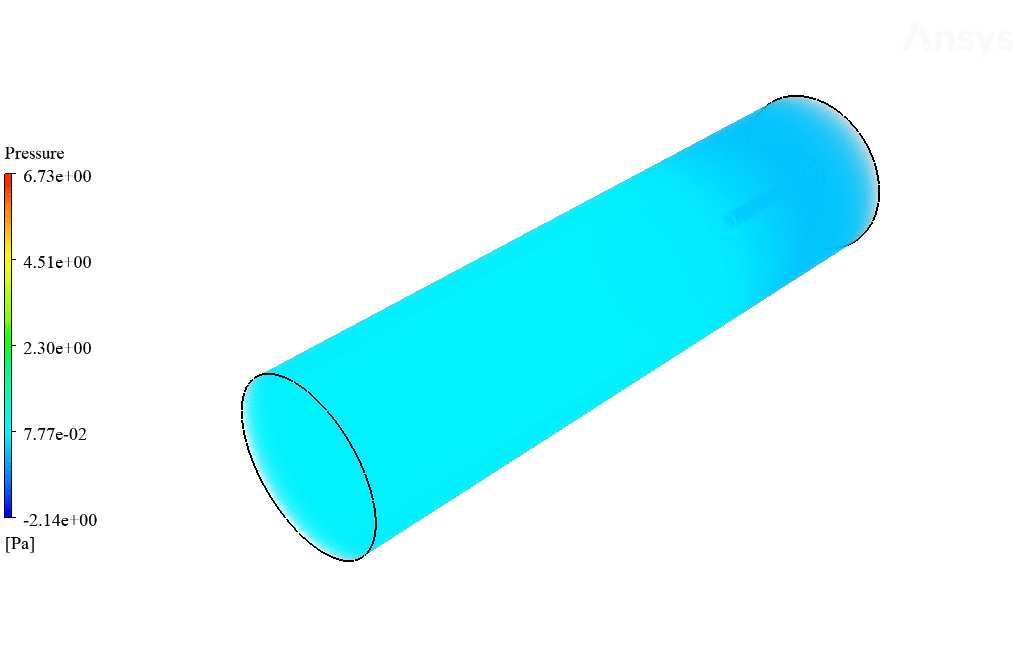
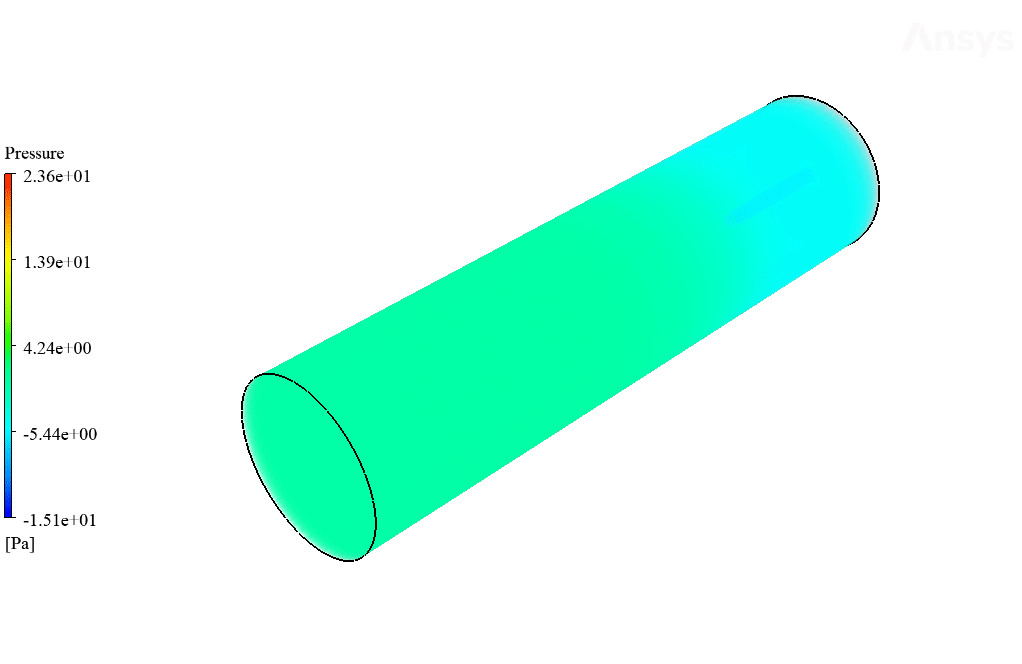
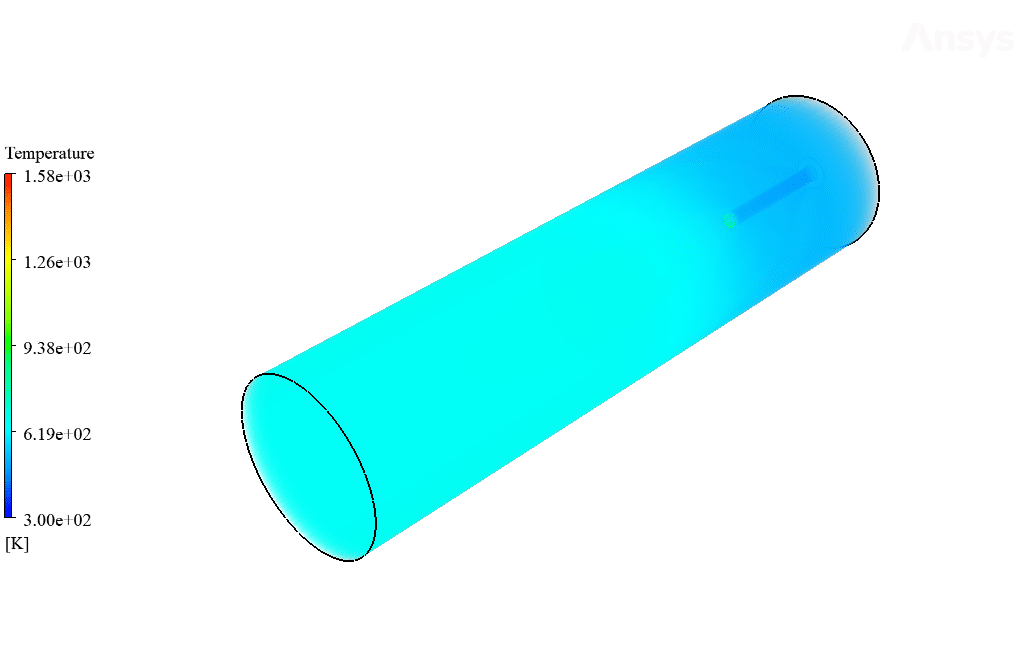
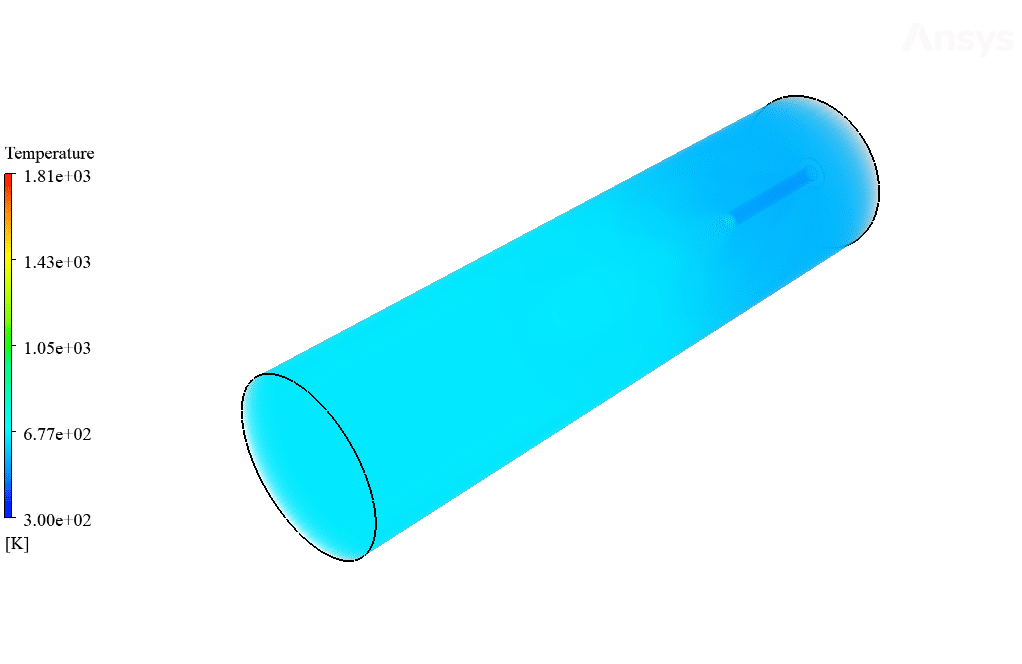
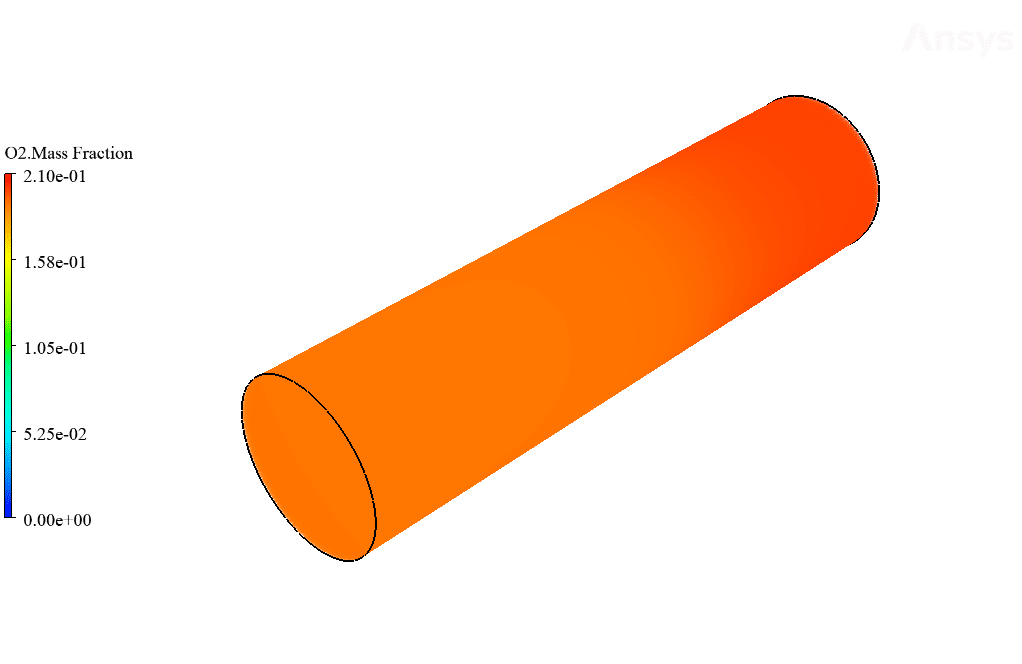
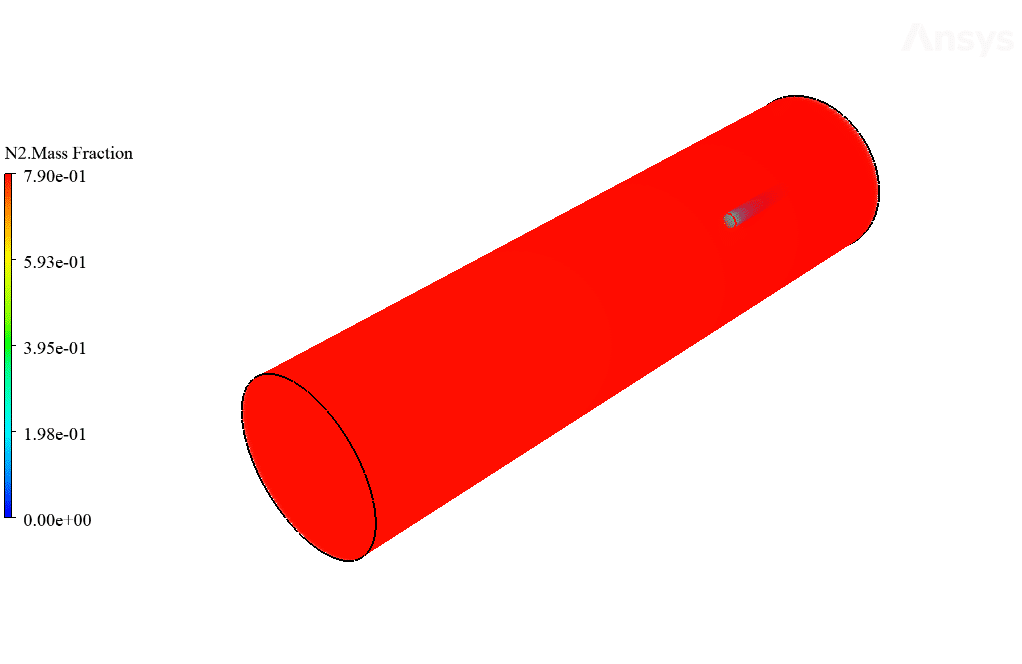
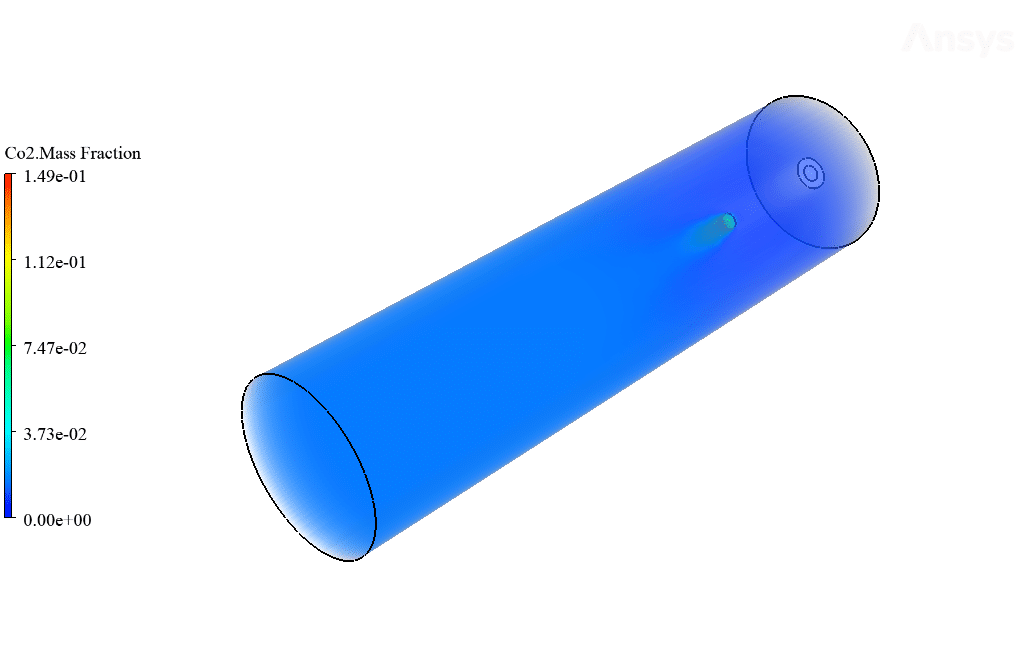
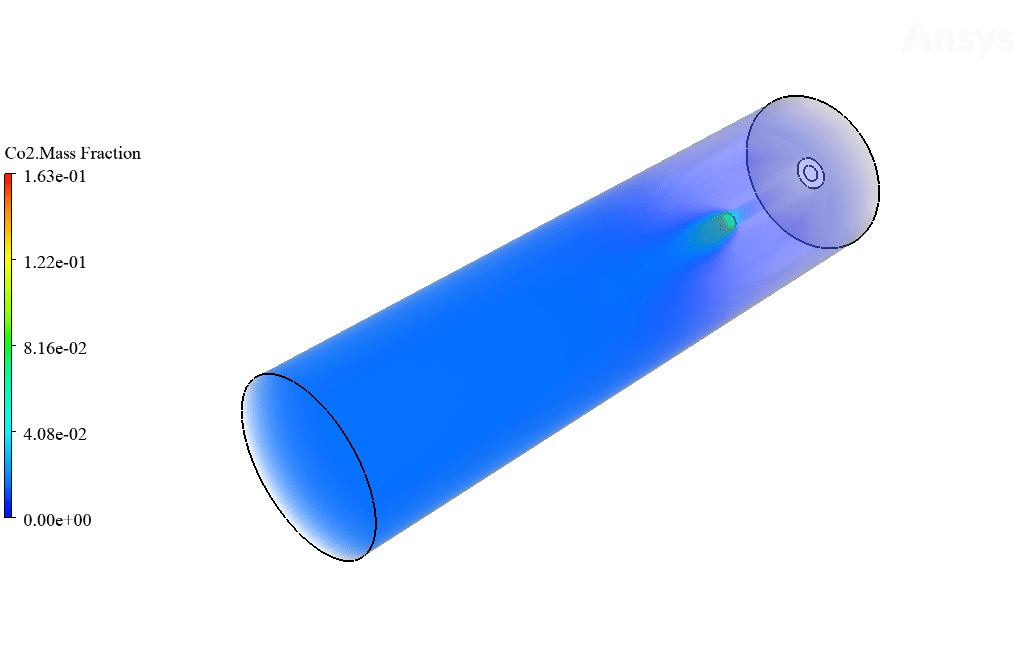
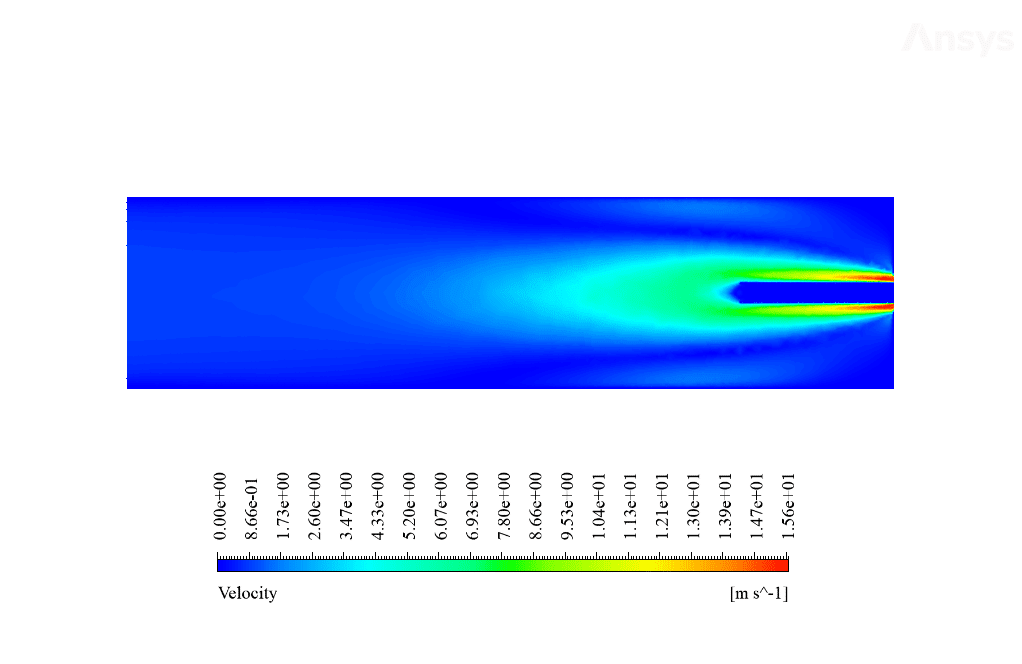
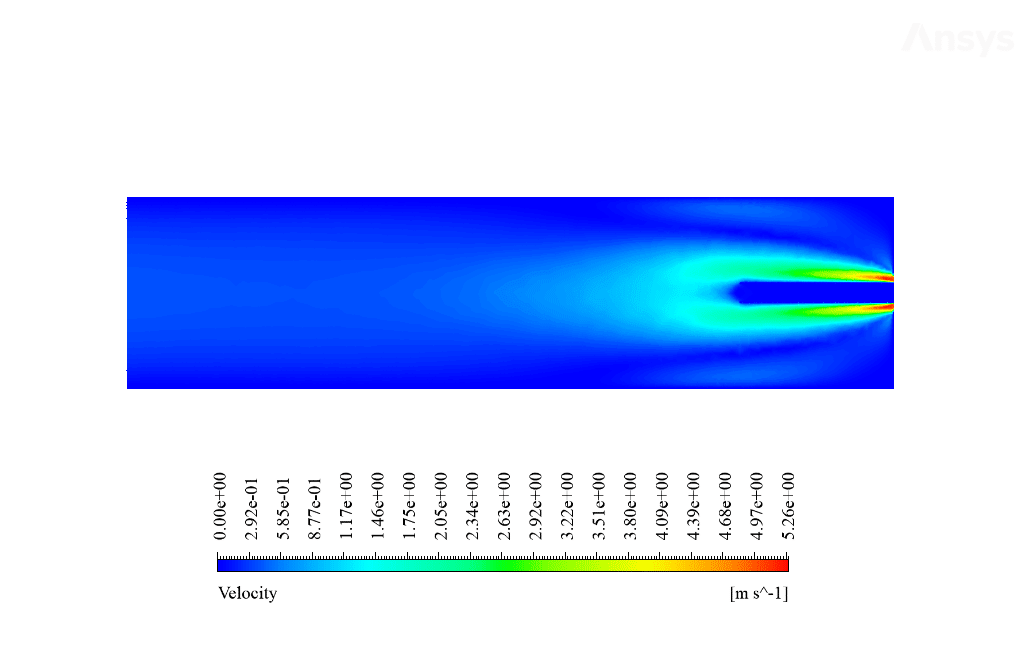
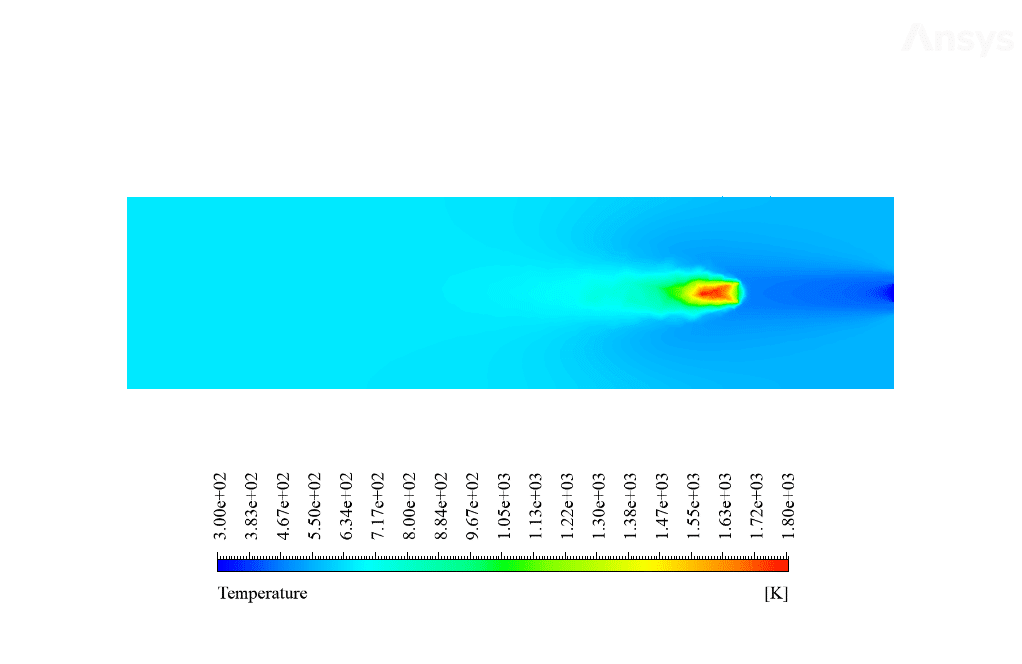
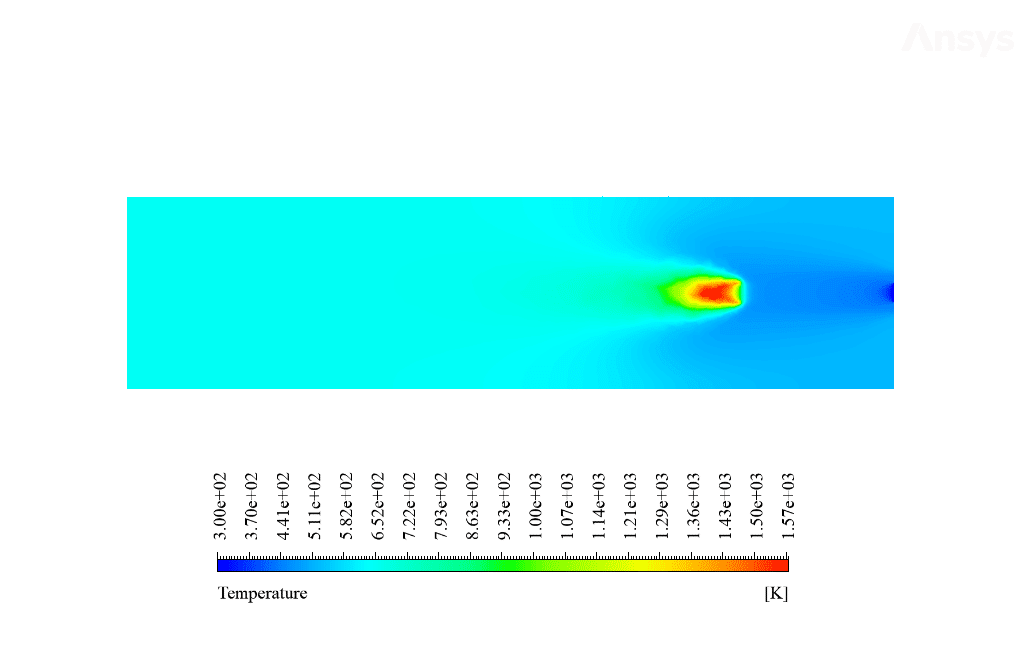
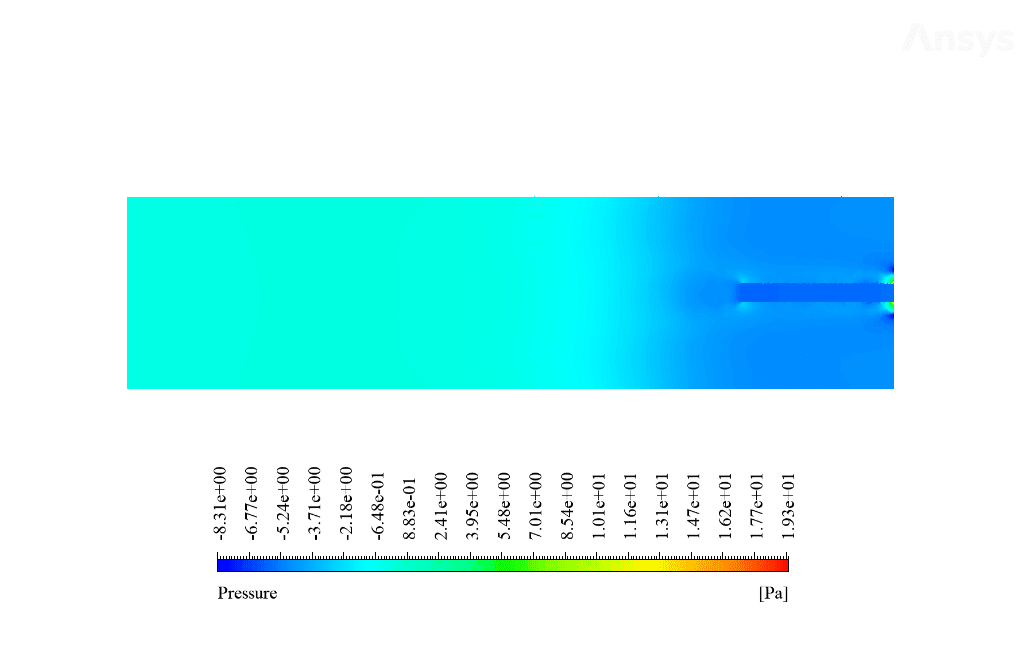
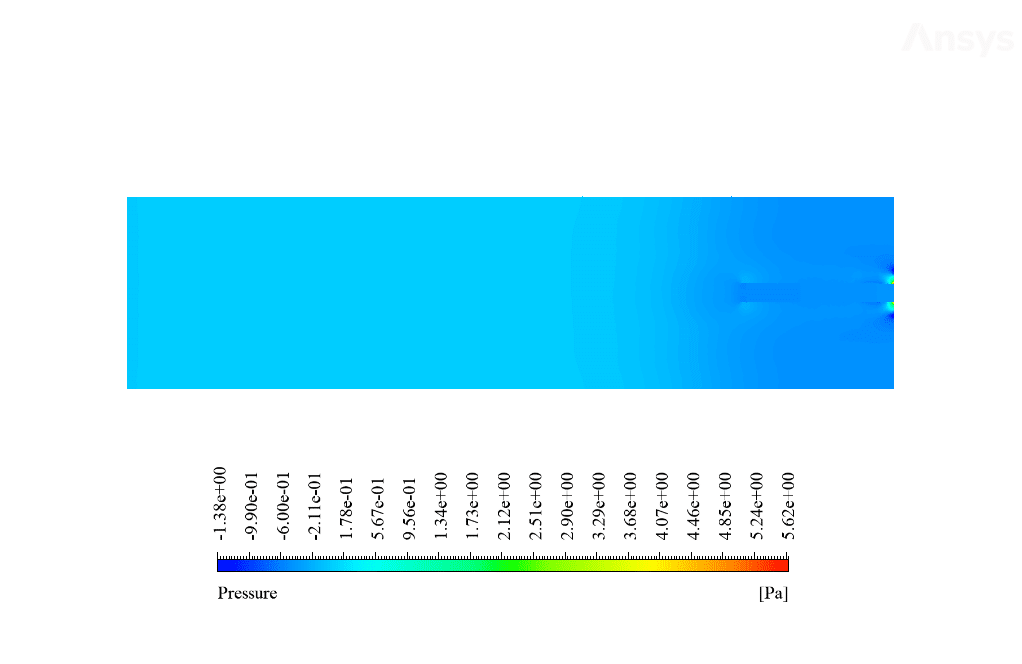
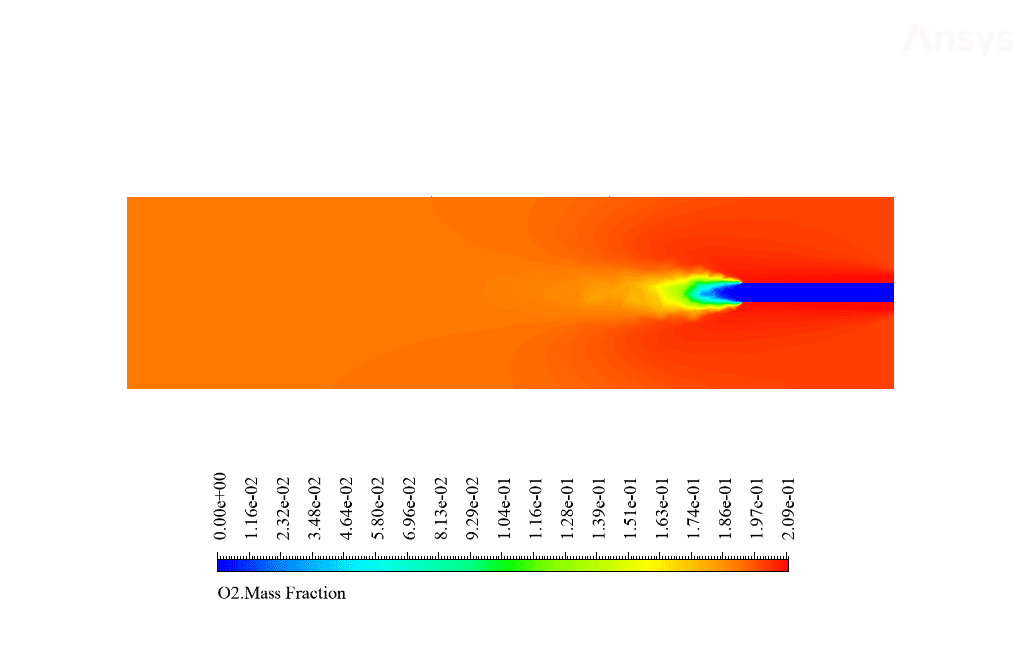
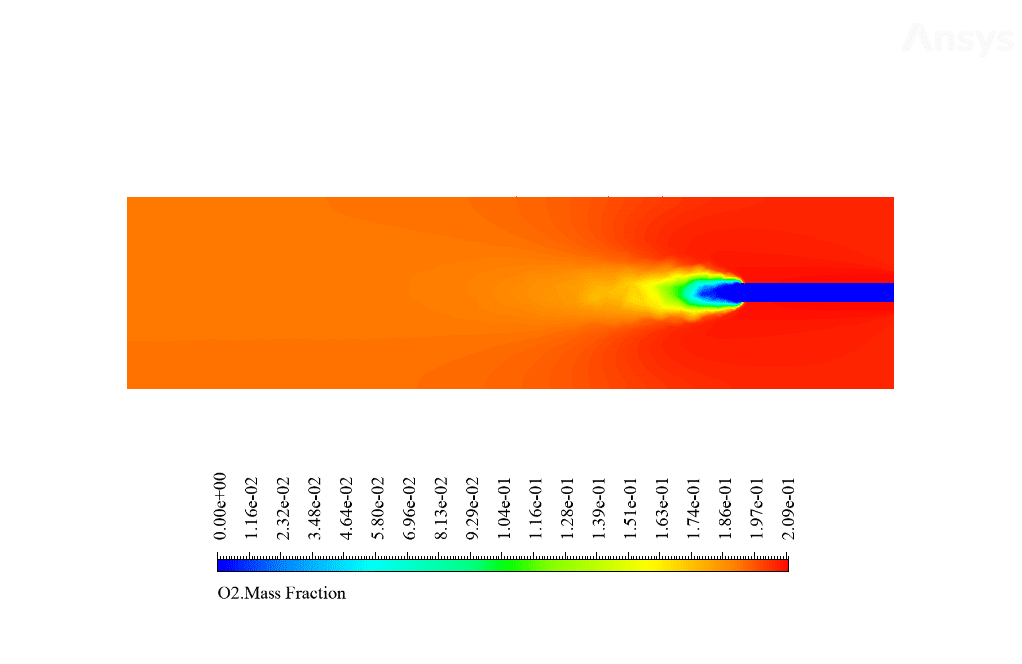
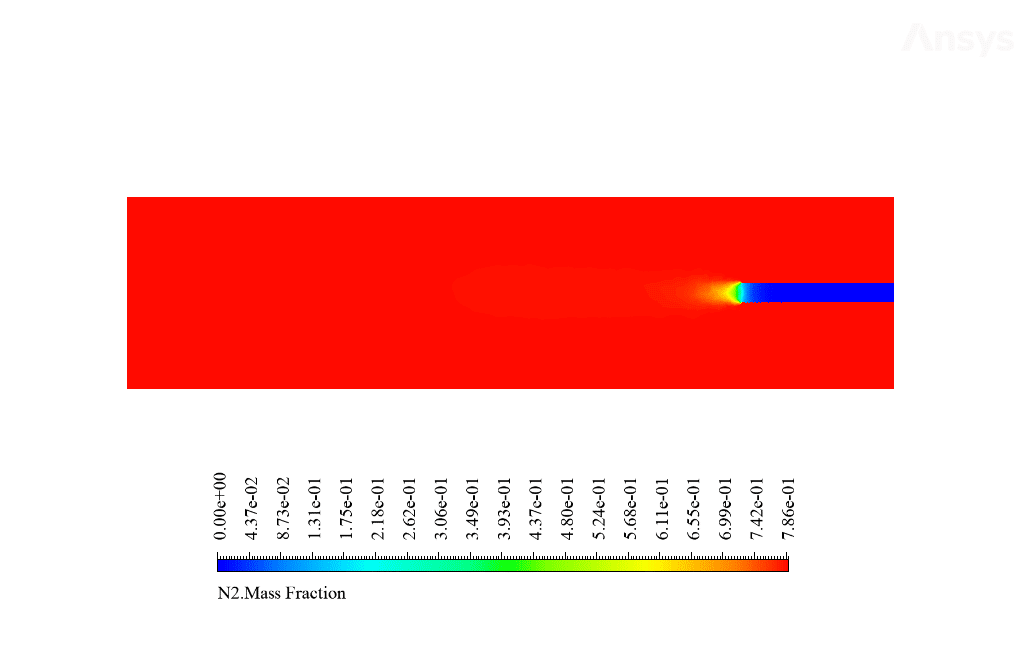
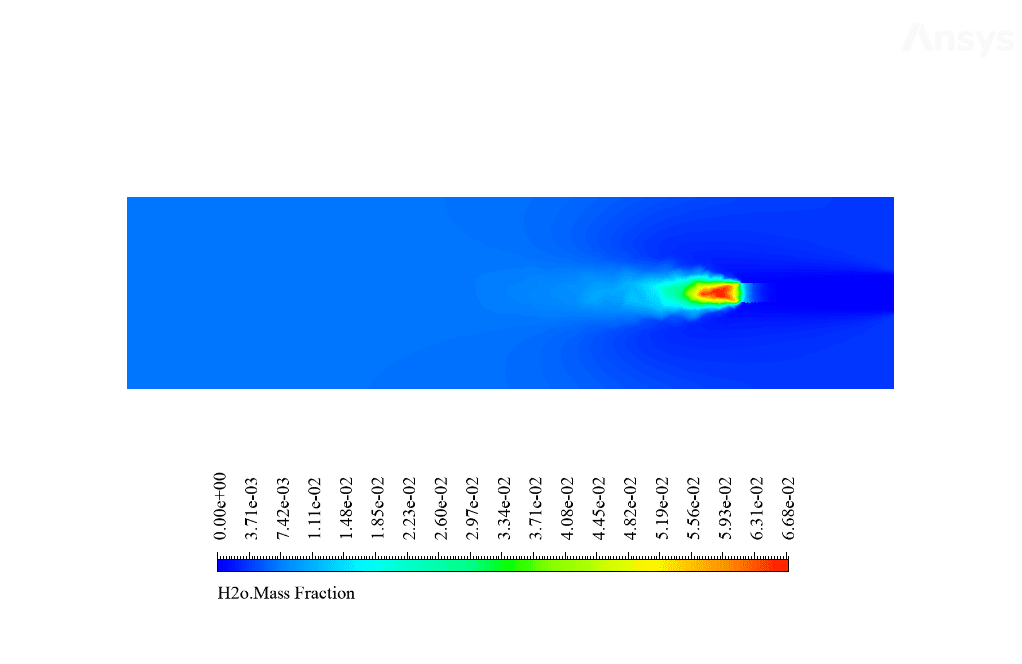
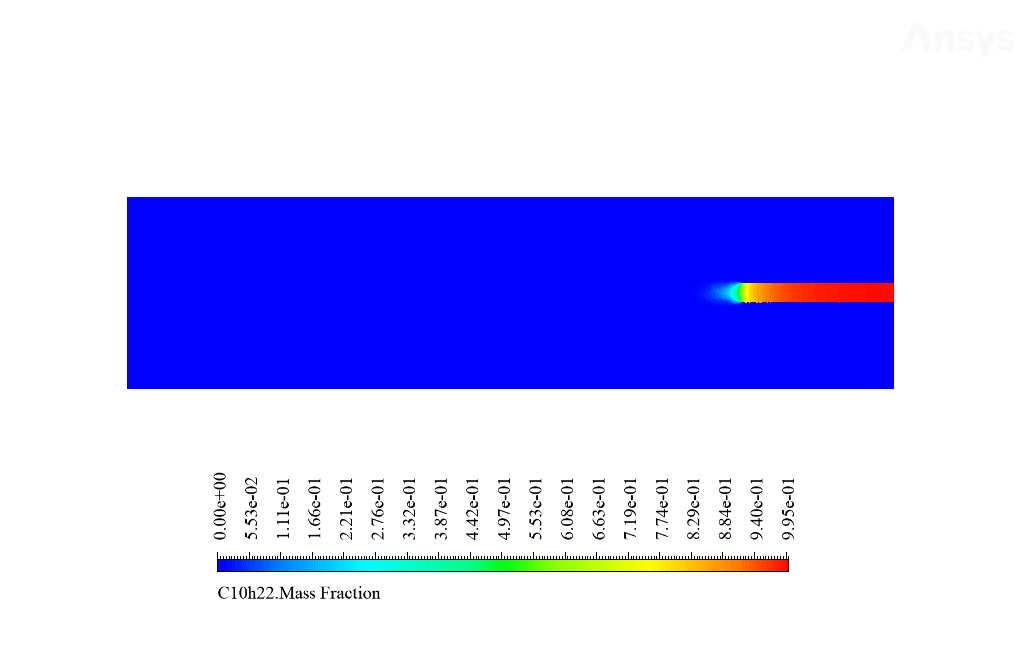
After calculation, 2D and 3D contours related to temperature, velocity, pressure, and mass fraction of species (CO2, C10H22, O2, N2, and H2O) are obtained. These results are displayed in two modes (in the absence of EHD and the presence of EHD) so that the effect of the electric field can be studied by comparing the results.
The contours show that when EHD is applied to the combustion chamber, more heat is applied to the species, resulting in higher product temperatures. Increasing the temperature of the reactive species causes the combustion reaction to occur more rapidly. Also, by examining the behavior of reaction products, it can result that the combustion reaction in the presence of EHD will be of higher quality.








Caleb Metz –
The technical detail provided in this review is impressive! The combination of species transport and EHD in a combustion process seems fascinating, and the analysis provides critical insights.
MR CFD Support –
Thank you! We’re delighted that the simulation results and the insight they provide catch your interest. Our goal is always to deliver comprehensive and informative simulations that enable deep understanding of the complex processes involved. If you have any further questions or require more information, please feel free to reach out.
Alexandrine Cartwright –
I really liked how the EHD model enhances the combustion process according to your results! Will applying higher current densities than 40 A/m^2 further increase the combustion efficiency, or is there an optimal range for the current density?
MR CFD Support –
Thank you for your kind words! As for your question, it is important to note that while increasing the current density can influence the combustion process and efficiencies, there is indeed an optimal range that must be considered to avoid any undesirable effects such as material degradation or electrical system safety concerns. This optimal range may vary depending on the specific setup and materials used in the combustion chamber. The outcome with higher current densities should be assessed with careful experimentation, considering the balancing factors of combustion efficiency and system integrity.
Cassie Aufderhar –
What specific impact does EHD have on the dynamics of combustion in the chamber based on the simulation results?
MR CFD Support –
According to the results of the simulation, the application of the EHD influences the combustion dynamics by generating more heat and raising the temperature of the reactants. This increase in temperature accelerates the combustion reaction, leading to a faster and more efficient combustion process. Higher quality combustion is achieved in the presence of EHD, as evidenced by examining the behavior of the reaction products.
Miss Christelle Jacobson IV –
Can you highlight the main benefits of applying EHD to the combustion chamber based on the simulation results?
MR CFD Support –
Absolutely! The simulation results suggest that when EHD is applied to the combustion chamber, there are key benefits such as increased levels of heat delivered to the reactive species, leading to higher product temperatures. This can cause combustion reactions to proceed more efficiently and rapidly. Additionally, the quality of the combustion reaction appears to be improved in the presence of EHD, potentially enhancing overall performance.
Mackenzie Flatley –
I absolutely loved the depth of information provided with this training on CFD simulation of combustion in the presence of EHD. Applying real scientific principles such as EHD in the simulation adds such an incredibly real touch. Thank-you MR CFD Company for an engaging training experience!
MR CFD Support –
Thank you for your positive feedback! We’re thrilled to hear that you found the training engaging and informative. Your appreciation for the scientific intricacies included in the CFD simulation means a lot to us. At MR CFD Company, we constantly strive to provide high-quality, realistic simulation experiences. We look forward to bringing you more valuable learning experiences in the future!
Keeley Schoen –
I’m fascinated by the EHD’s role here. Could you explain how it influences the combustion process in more simple terms?
MR CFD Support –
Of course! In simple terms, EHD, or electrohydrodynamic, introduces an electric field to the combustion area. This electric field causes charged particles or molecules in the fuel to move and interact with both the field and the airflow around them. This interaction can improve mixing of fuel and air, which can make the combustion more efficient. As a result, there is better fuel burn, more heat release, and a higher combustion quality.
Elwyn Fisher –
The explanation of the combustion with EHD is very clear. The comparison of the chamber’s operation with and without the EHD influence provides a thorough understanding of the benefits and changes that EHD introduces to combustion processes.
MR CFD Support –
Thank you for your kind words. We’re delighted to hear that you found our tutorial on Combustion in the Presence of EHD to be thorough and insightful. Understanding the influence of EHD on combustion helps optimize industrial processes, and we are glad that our material could elucidate this complex subject for you. Your interest and satisfaction are very important to us!
Era Terry –
I’m truly impressed with the thoroughness of the EHD combustion simulation. It was fascinating to learn how the electric field can enhance the combustion process. Great job on designing the simulation and clearly presenting how EHD influence may lead to more efficient combustion. The results surely provide valuable insights for real-world applications. Keep up the excellent work!
MR CFD Support –
Thank you for your kind words! We’re really glad to hear that you found the simulation informative and insightful. It’s wonderful to know that the detailed setup and presentation of results were helpful in understanding the impact of EHD on the combustion process. We appreciate your positive feedback and encouragement to continue providing high-quality simulations.
Ahmed Rowe –
Can you please clarify the overall impact of EHD on combustion efficiency based on the simulation results?
MR CFD Support –
Certainly! The simulation results suggest that the application of Electrohydrodynamic (EHD) leads to an increase in the reactive species’ temperature. This induced heat promotes more rapid combustion reactions, thereby improving efficiency. The enhanced reaction rates suggest that EHD technology could be leveraged to achieve higher-quality combustion within the chamber.
Keara Becker –
The combination of simple terms and detailed explanations made this review comprehensive without overwhelming the reader. Well done on mentioning the impact of EHD on combustion quality.
MR CFD Support –
Thank you for your positive feedback! We are thrilled to hear that the explanation of EHD’s role in enhancing combustion quality was clear and informative. Your satisfaction is our top priority, and we look forward to providing you with more quality training materials!
Clovis Graham –
I’ve learned so much from this training on combustion in the presence of EHD! The level of detail in explaining the species transport model was spot-on. It was fascinating to see the impact of EHD in enhancing the combustion process. The visuals for temperature and velocity contours made the complex concepts much more understandable. Excellent resource for anyone looking into advanced combustion simulation.
MR CFD Support –
We’re thrilled to hear that our training on combustion with EHD in ANSYS Fluent was informative and valuable to you! It’s great that the visuals were helpful in understanding the concepts. Thank you for your positive feedback; it motivates us to continue providing high-quality learning resources!
Maybelle Toy IV –
The explanation of the CFD simulation incorporating EHD in the combustion process is fantastic! It clarified the impact of the electric field on the combustion efficiency and the temperature of the species within the chamber. The utilization of visuals in the form of 2D and 3D contours undoubtedly aids in understanding the intricate interplay between the electric field and combustion processes.
MR CFD Support –
We are thrilled to hear that you found the simulation materials comprehensive and enlightening! Visual tools like our 2D and 3D contours are indeed instrumental in grasping the complexities of such intricate phenomena. Thank you for recognizing the vitality of our simulation approach in enhancing understanding. If you ever have any more questions or need further insights, feel free to reach out to us.