Wet Combustion Using DPM Combusting Particle
$180.00 Student Discount
- The problem numerically simulates the combustion chamber by DPM combusting particle type.
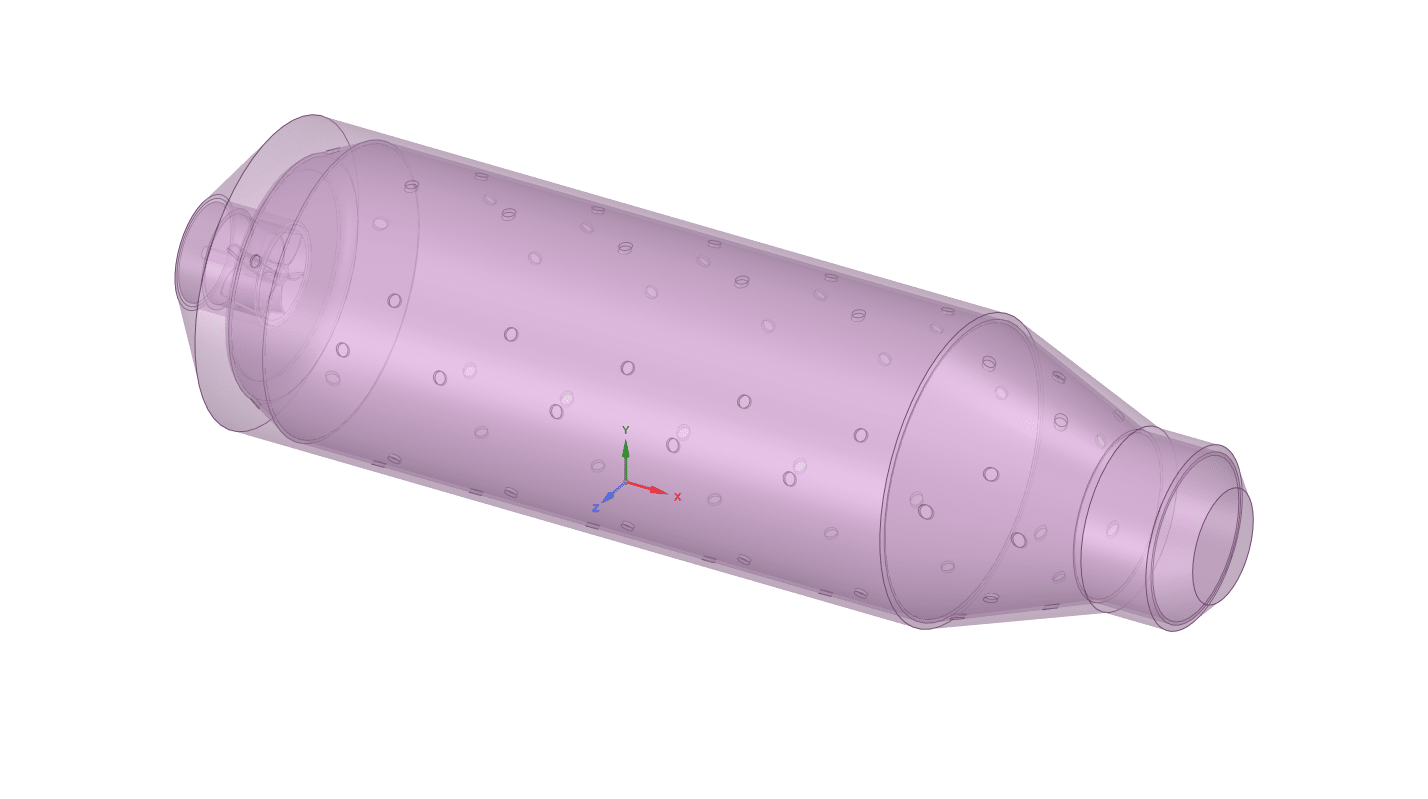
- We design the 3-D model by the Design Modeler software.
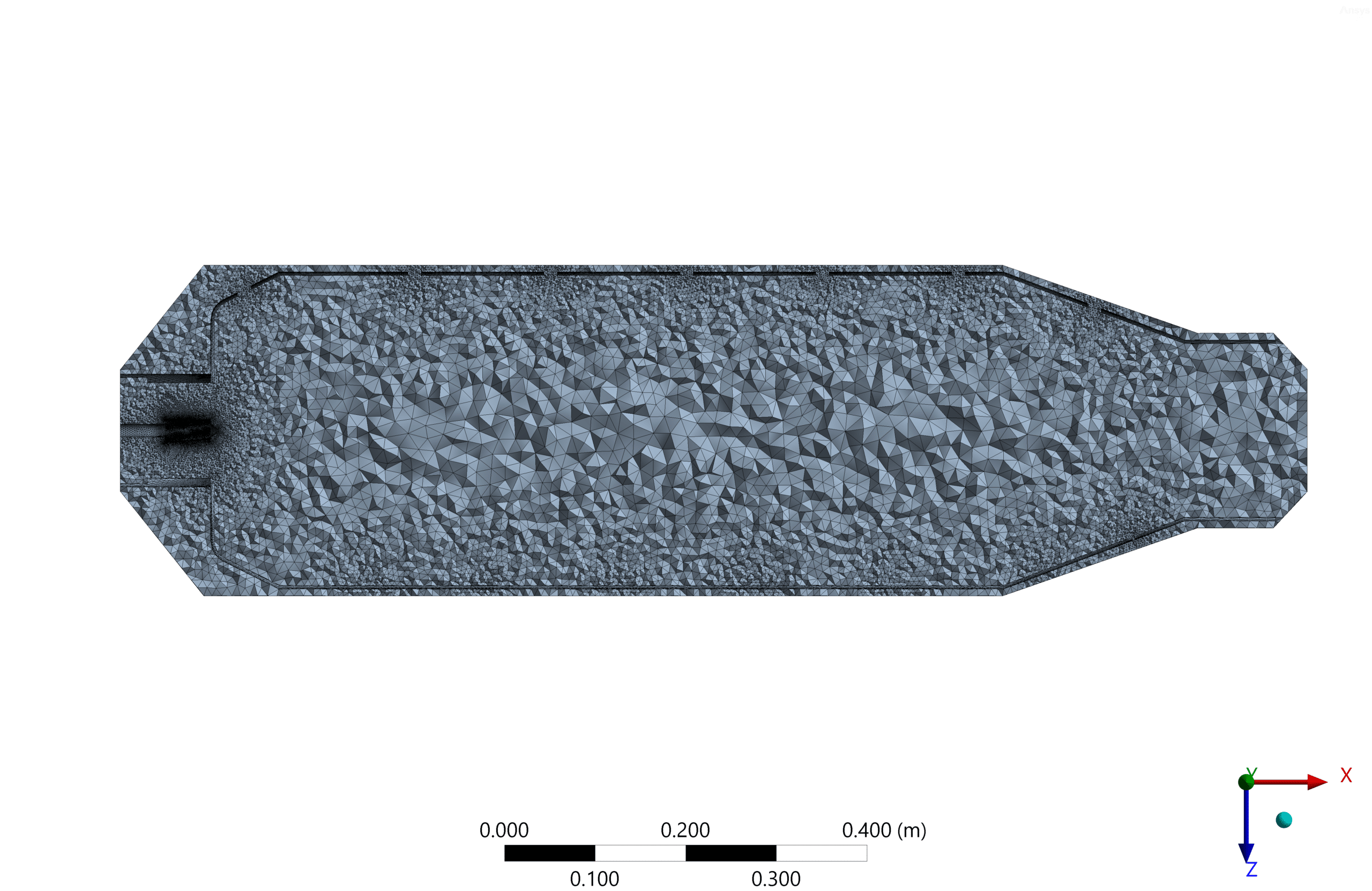
- We Mesh the model by ANSYS Meshing software.
- We perform this simulation as unsteady (Transient).
- We use the Species Transport & DPM model to define the combustion process.
To Order Your Project or benefit from a CFD consultation, contact our experts via email ([email protected]), online support tab, or WhatsApp at +44 7443 197273.
There are some Free Products to check our service quality.
If you want the training video in another language instead of English, ask it via [email protected] after you buy the product.
Description
Project Description:(Wet Combustion in a Combustion Chamber Using DPM, Combusting Particle)
In this project, the wet combustion of anthracite volatile in a combustion chamber using combusting particles is simulated. We aim to track fuel particles from the beginning of devolatilization, oxidizing and producing carbon dioxide & water vapor.
It should be mentioned that the liquid fraction of the fuel is 2%, and the injection lasts for 0.5 seconds. Meanwhile, hot air enters the combustion chamber, increasing the temperature of the particles and causing the volatile fraction to be released.
This product is the 4th chapter of the DPM training course.
Methodology of Wet CombustionUsing DPM Combusting Particle:
Species transport and discrete phase model (DPM) have been used to simulate anthracite particles. The combusting particle type is hired with a 2% liquid fraction. The initial temperature of particles is 325K with a nonspherical shape and rosin-rammler diameter distribution.
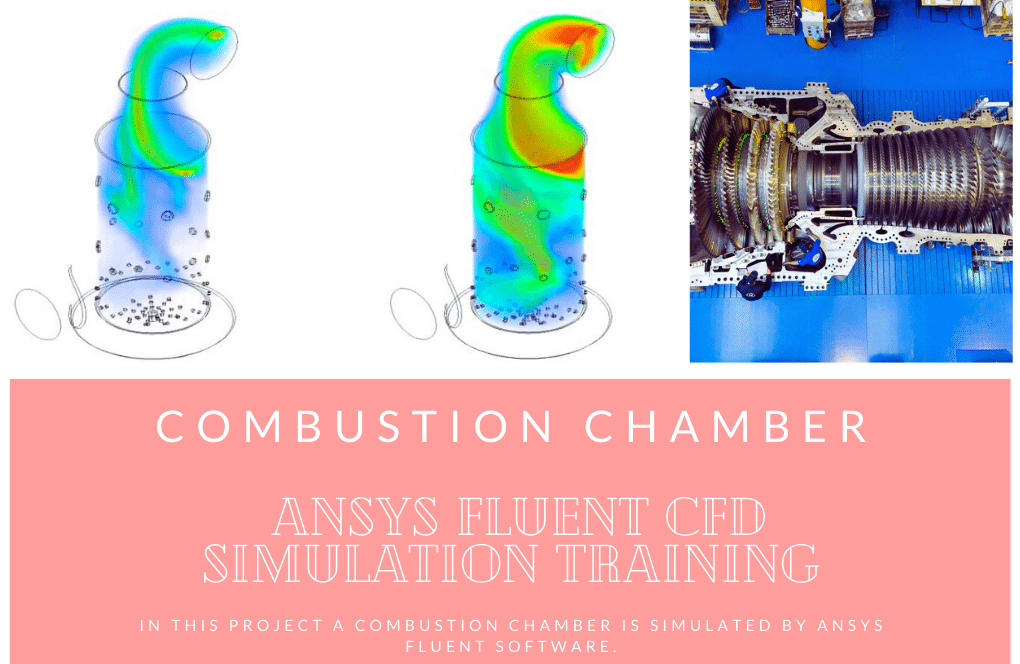
The geometry is designed using ANSYS Design Modeler software. In addition, an unstructured mesh grid is generated using ANSYS Meshing software. In the next step, they converted them to polyhedron cells to reduce computational costs. As a result, 810000 cells were generated.(check figures below)
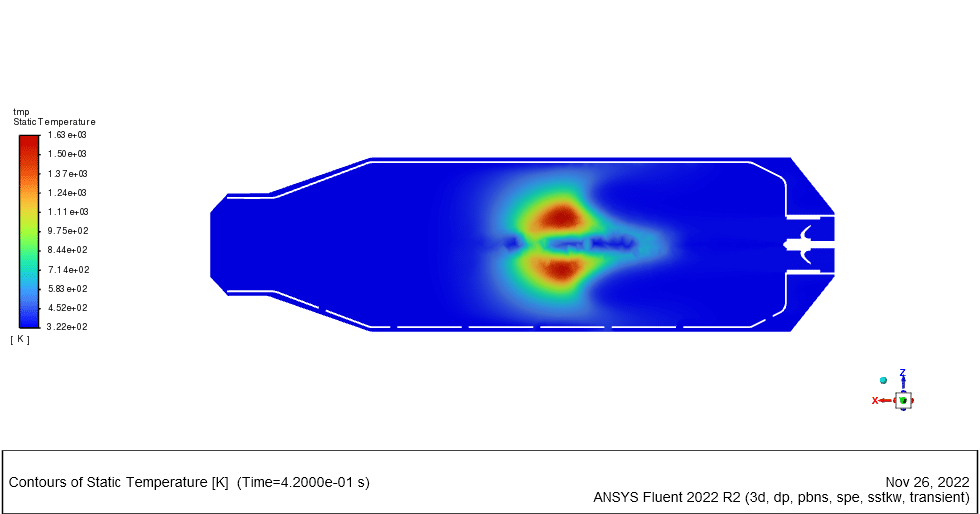
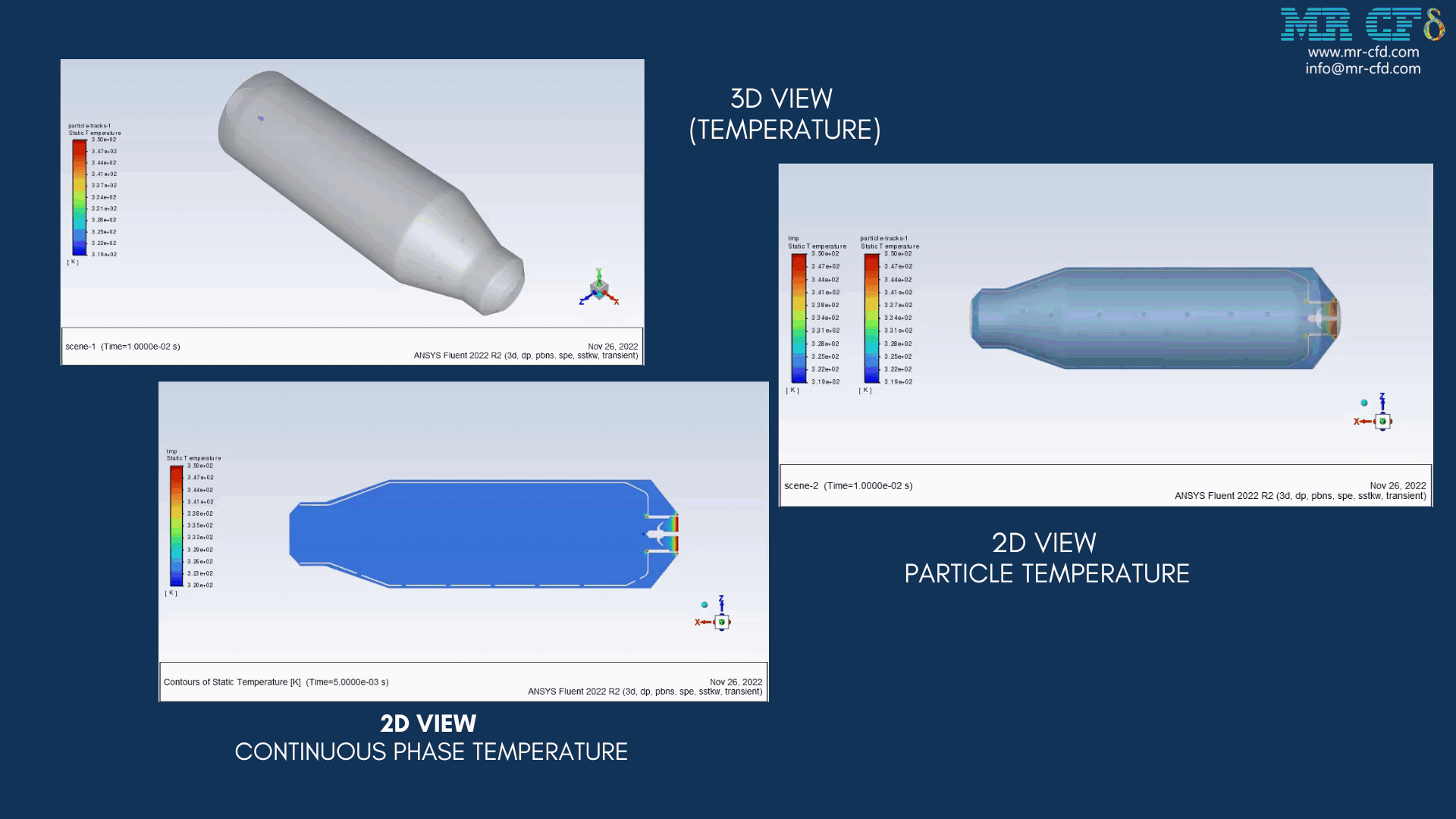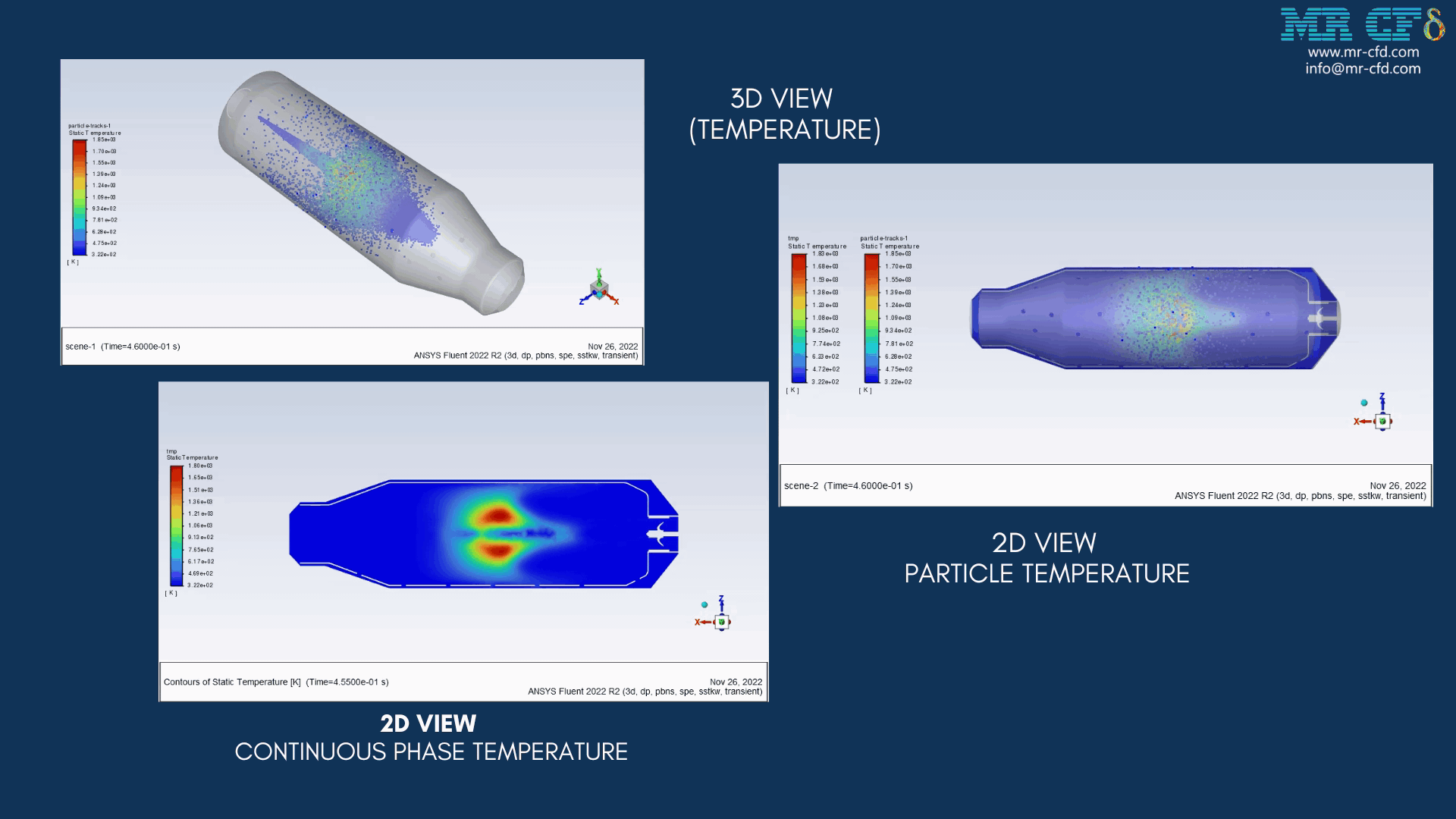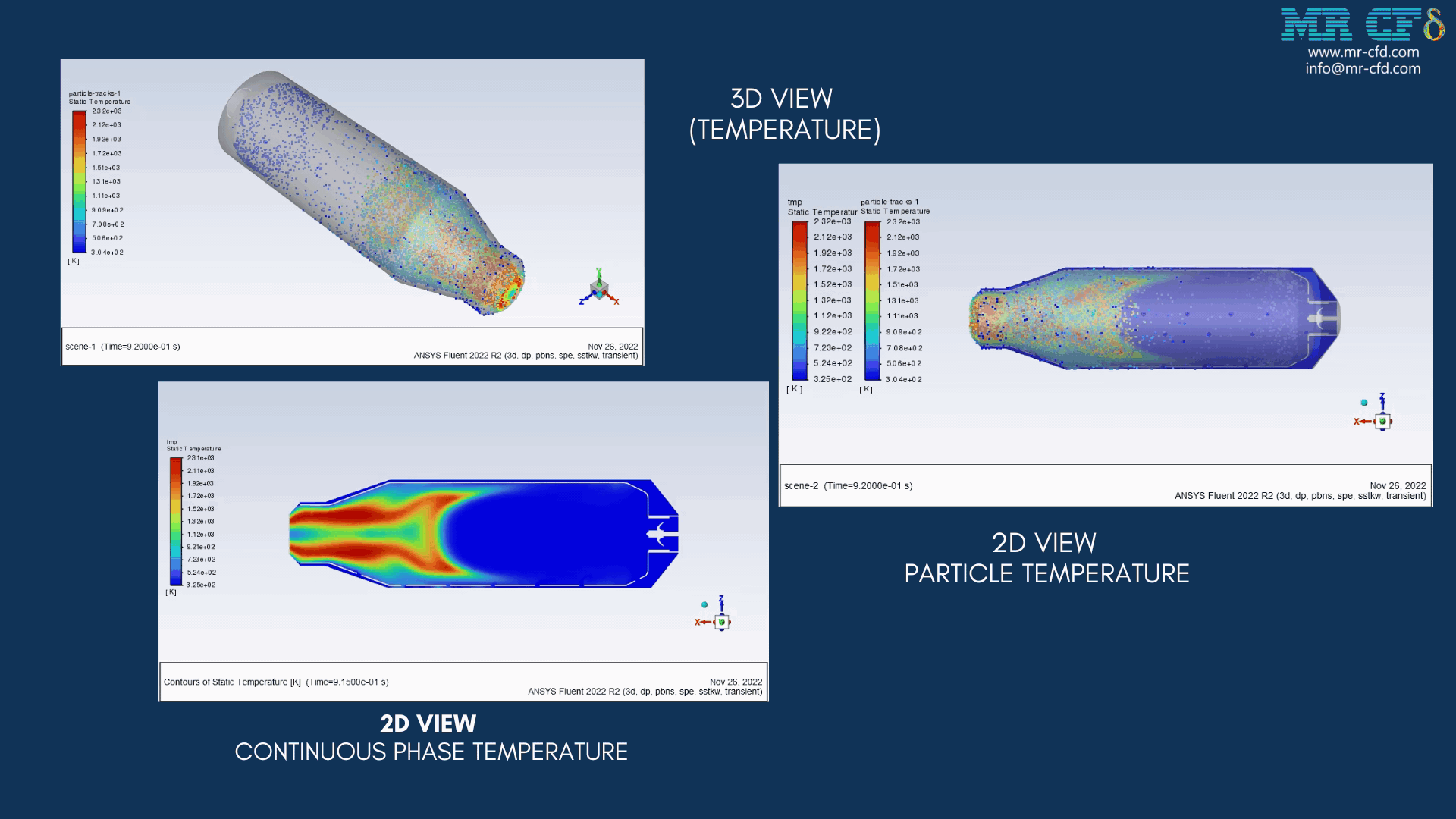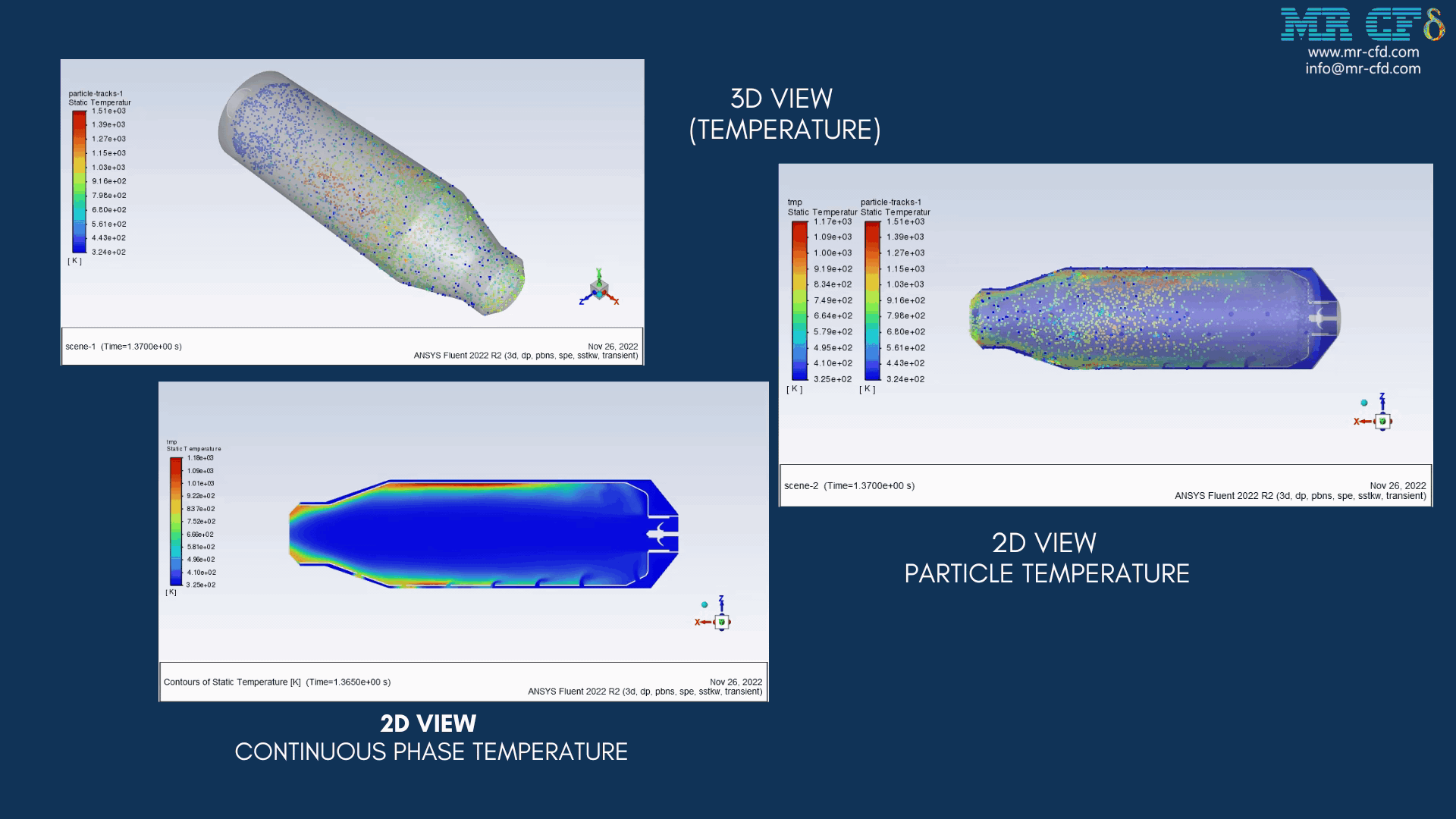
Results of wet combustion:
As the injection starts in the combustion chamber, the wet part of the anthracite starts evaporating. Although there is enough oxygen in the domain, the reaction doesn`t occur until the volatile fraction is released. The volatile fraction oxides and produces carbon dioxide and water vapor:
1An-vol + 2.207 O2 ==> 0.1 Co2 + 4.408 H2o
The energy released from the reaction increases the chamber temperature up to 2400K. For better illustration, animations are extracted which can be seen below.
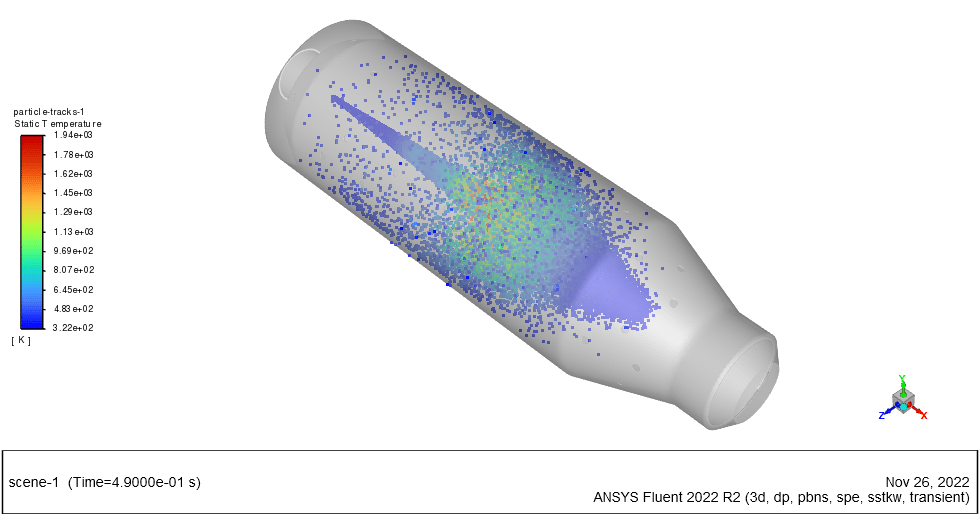
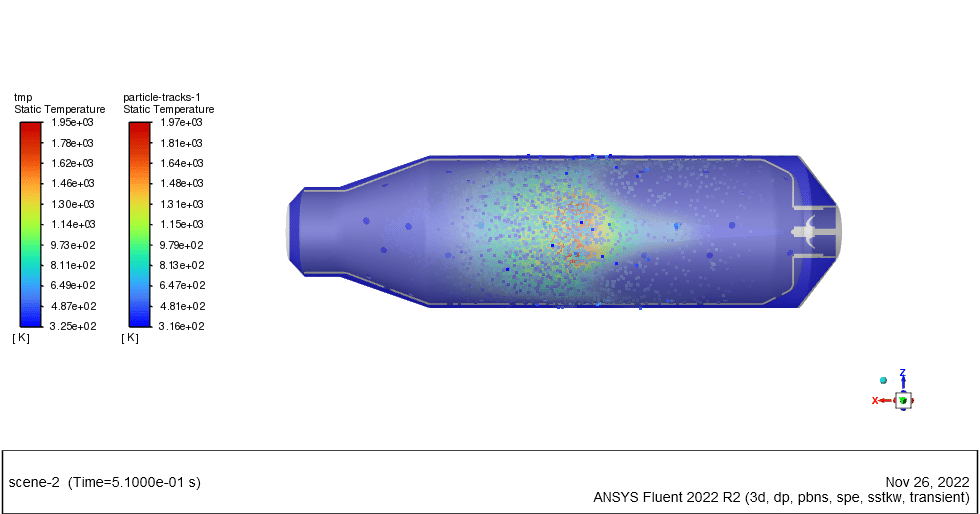
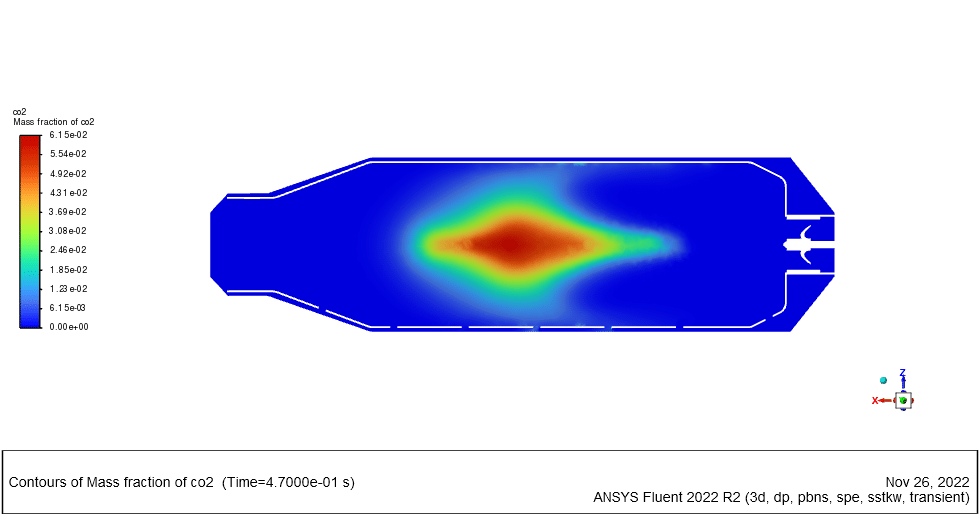

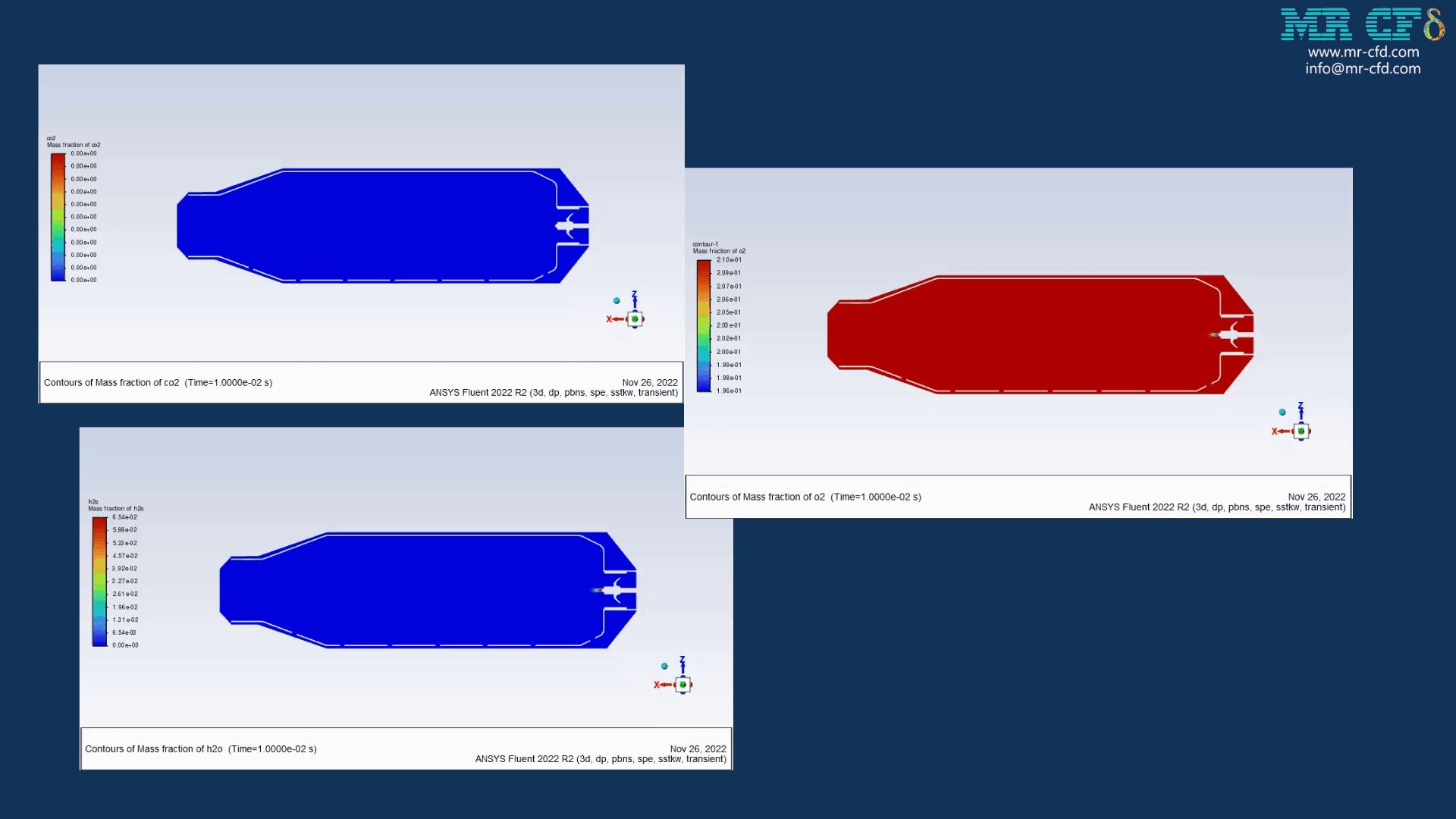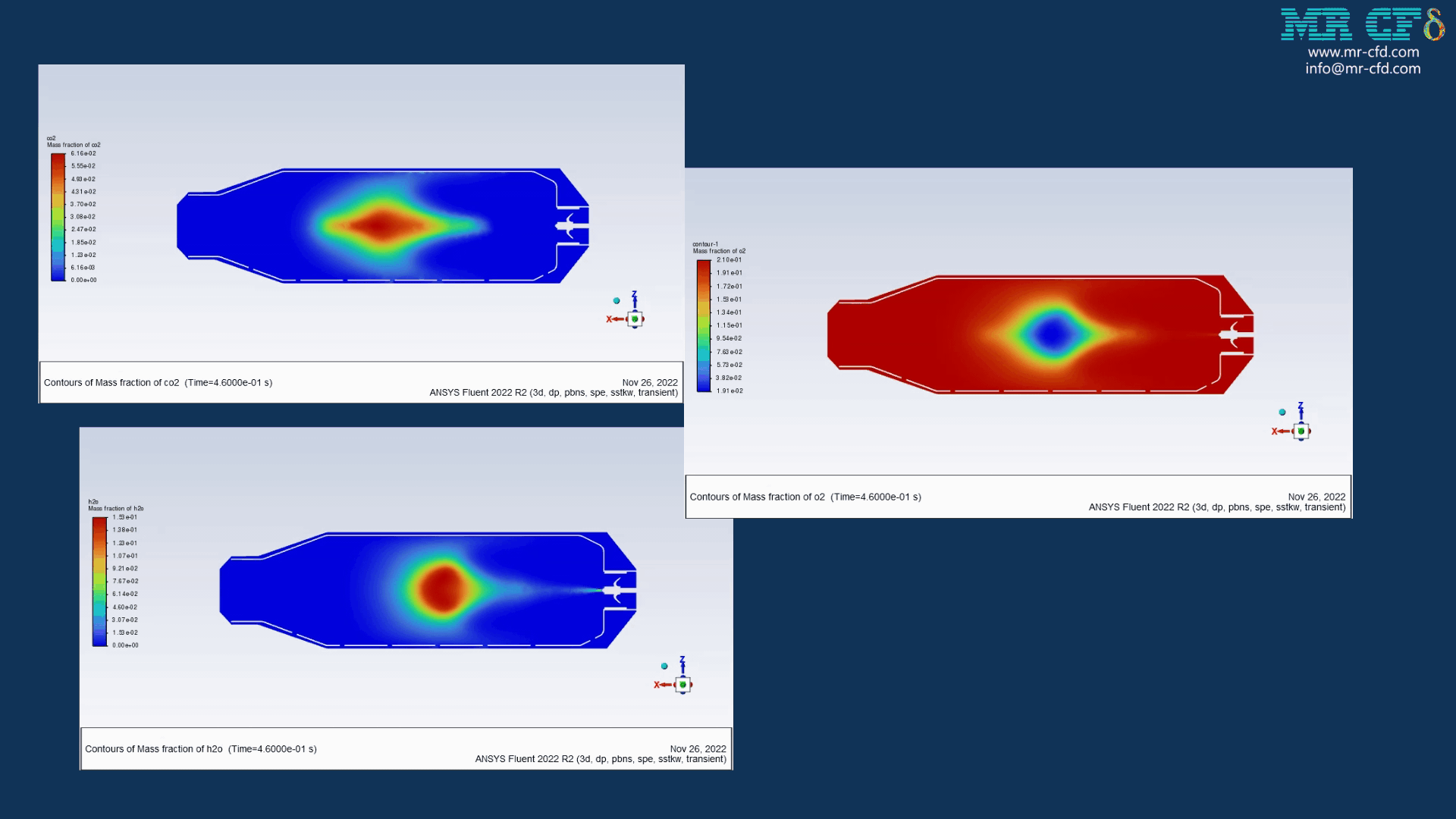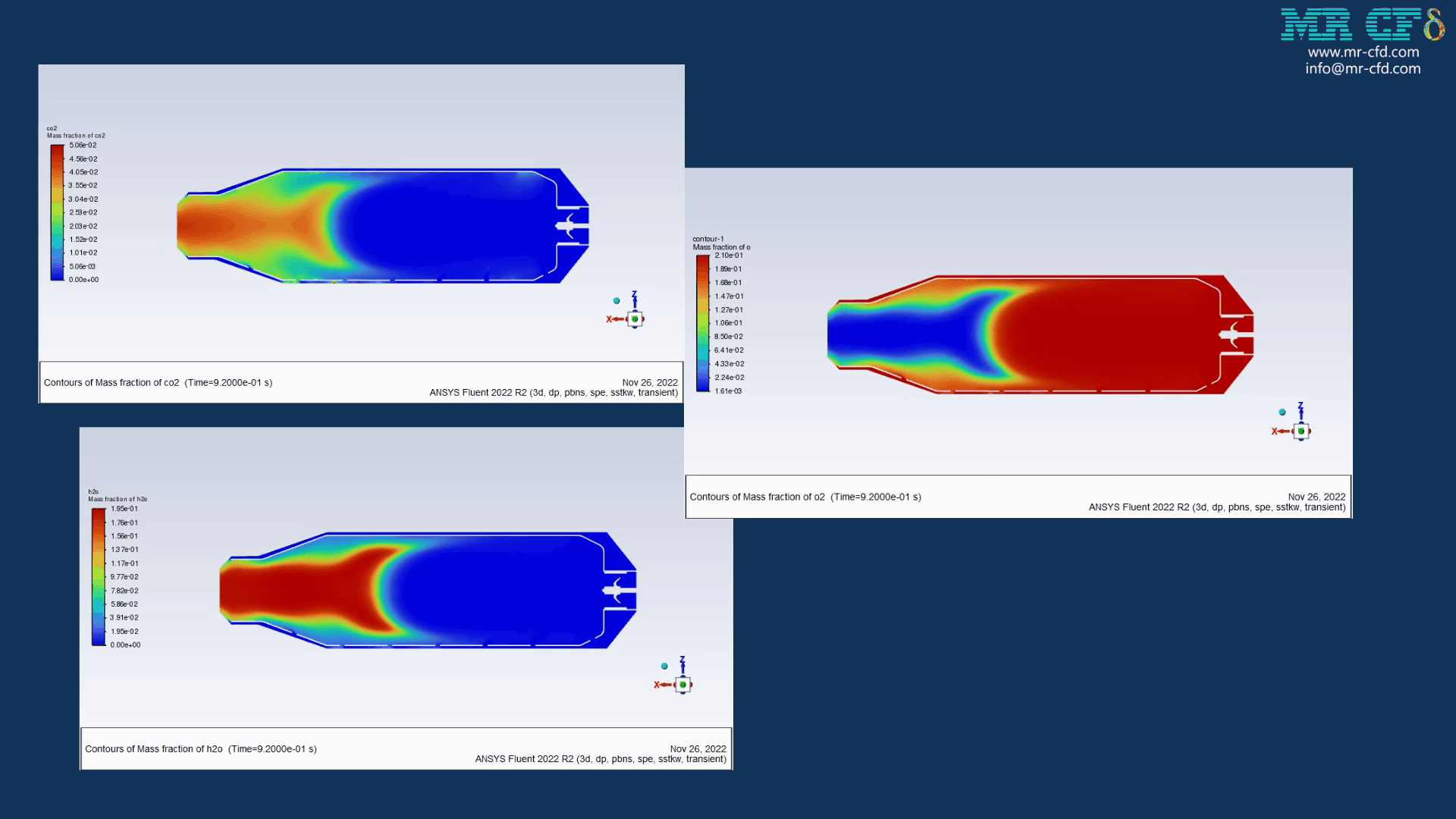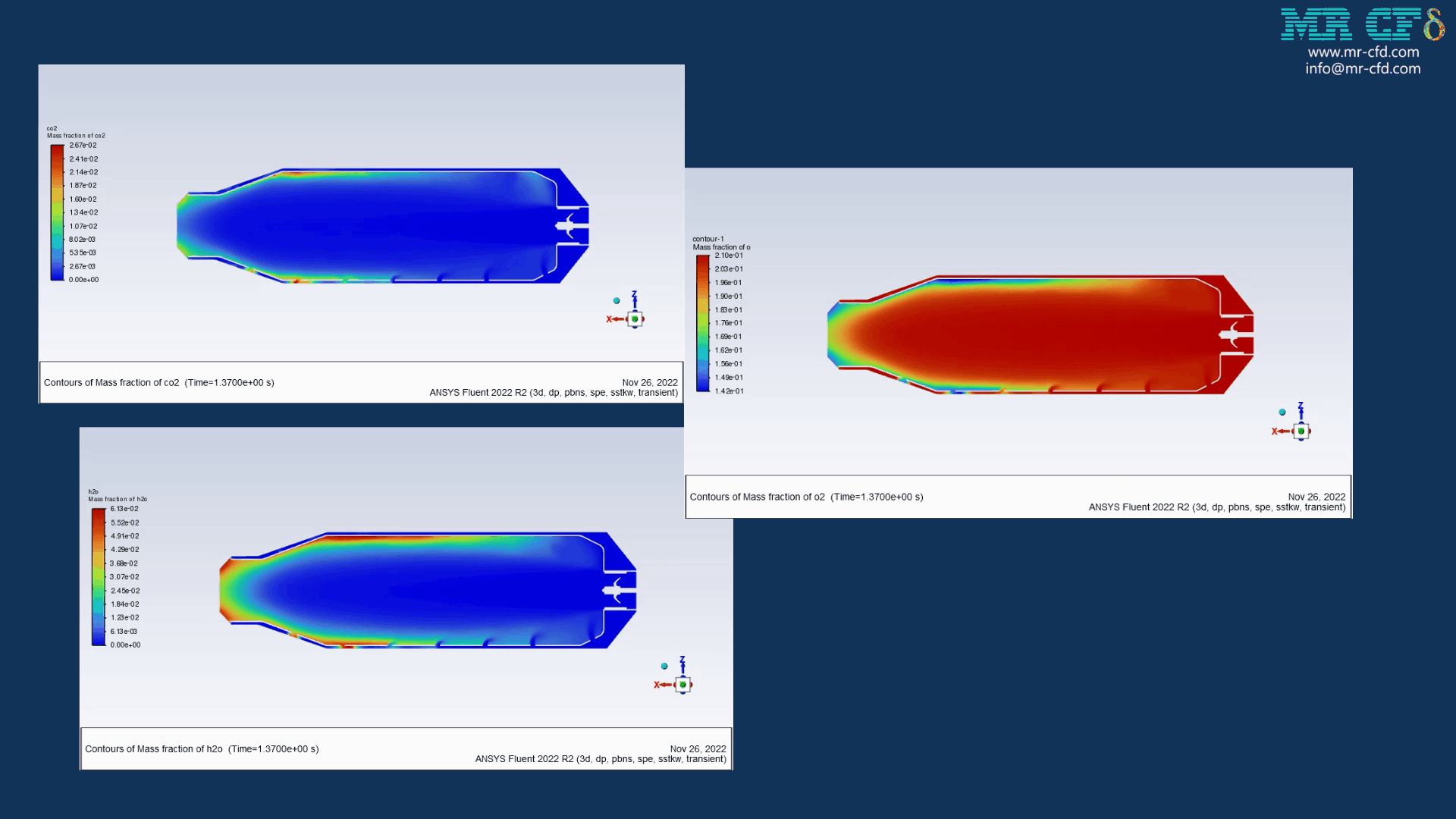

Minerva Carroll –
Can you tell me more about how the liquid fraction of the fuel is modeled in this simulation?
MR CFD Support –
In the simulation, the liquid fraction of the anthracite fuel particle is set to 2%. This is achieved using the Discrete Phase Model (DPM), wherein the particle properties can be adjusted to include the specific wetness content. During the simulation, as the hot air heats up the particles, the liquid fraction evaporates first, followed by the devolatilization of the solid part, thereby releasing the volatile components that react with the oxygen. This phenomenon is modeled by specifying the properties of the fuel particles, including their size distribution, initial temperature, shape, and composition, particularly noting the liquid content which directly impacts the combustion process.