Decomposition of MgO with Argon Gas for Magnesium Particle Production, ANSYS Fluent Training
$140.00 Student Discount
In this project, the decomposition of MgO with argon gas for magnesium particle production is simulated.
Click on Add To Cart and obtain the Geometry file, Mesh file, and a Comprehensive ANSYS Fluent Training Video.To Order Your Project or benefit from a CFD consultation, contact our experts via email ([email protected]), online support tab, or WhatsApp at +44 7443 197273.
There are some Free Products to check our service quality.
If you want the training video in another language instead of English, ask it via [email protected] after you buy the product.
Description
Decomposition Introduction
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. According to the following equation, the decomposition reaction of magnesium oxide is endothermic, and this process is done by preheating through argon gas.
MgO(s)→Mg(s)+O2(g)
Decomposition Problem Description
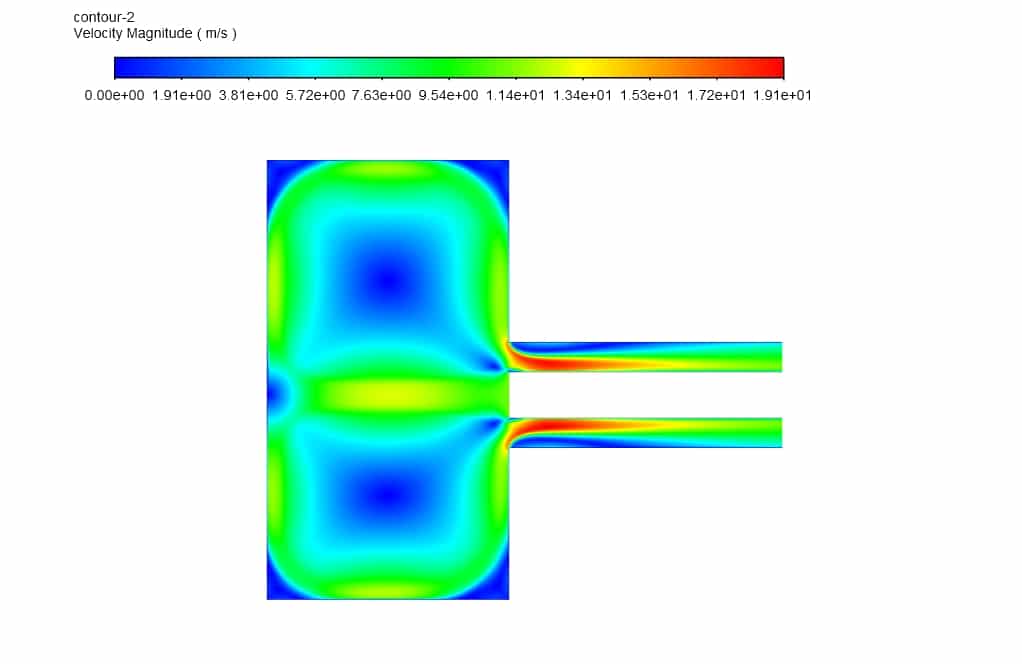
In this study, according to the geometry, the design and grid generation has been done. The combination of DPM (discrete particle method) and species transport methods was used to investigate the decomposition effect of magnesium oxide particles. The thermodynamic properties of the particles, along with the necessary boundary conditions, were applied in the settings. This reaction is exothermic in the reactor chamber and increases its temperature. From the left inlet, argon gas enters at a temperature of 300 ° C. From the right inlet, argon gas enters at a temperature of 700 ° C with the injection of magnesium oxide particles at the same temperature.
Geometry & Mesh
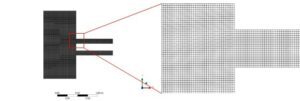
Geometry was designed in Design Modeler software, and the computational domain includes a square with dimensions of about 2 * 2 meters.
Ansys Meshing software was used for grid generation, and Structured mesh with about 53,000 elements has been used. Minimum Orthogonal Quality and Maximum Aspect Ratio were 0.9 and 2.6, respectively.
Decomposition Solver Setting
ANSYS Fluent software was used to solve the governing equations numerically. The problem is analyzed steady using the pressure-based method, and the gravitational effects were not considered. Also, for solving the above problem, RANS Includes discrete phase particles by integrating the force balance on the particles, which is written in a Lagrangian reference frame. This force balance equates the particle inertia with the forces acting on the particle. Species transport equations were also considered in the Navier-Stokes equations to exert the effect of the decomposition process.
Material Properties
All thermodynamic properties of the materials in this study are assumed to be constant.
Boundary conditions and Solution methods
Also, The table below shows the characteristics and values of boundary conditions, along with the models and hypotheses.
| Material Properties | |||
| Oxygen | |||
| Amount | Fluid properties | ||
| 1.2999 | Density (kg/m3) | ||
| Piecewise-polynomial | Specific heat (j/kg.K) | ||
| 0.0246 | Thermal conductivity (W/m.K) | ||
| 1.919e-05 | Viscosity (kg/m.s) | ||
| Argon | |||
| Amount | Fluid properties | ||
| 1.6228 | Density (kg/m3) | ||
| 520.64 | Specific heat (j/kg.K) | ||
| 0.0158 | Thermal conductivity (W/m.K) | ||
| 2.125e-05 | Viscosity (kg/m.s) | ||
| Droplet-particle (Mgo) | |||
| Amount | Fluid properties | ||
| 3540 | Density (kg/m3) | ||
| 880 | Specific heat (j/kg.K) | ||
| 30 | Thermal conductivity (W/m.K) | ||
| 1.919e-05 | Viscosity (kg/m.s) | ||
| Discrete phase model (DPM) | |||
| Interaction with continuous phase | 10 continues phase iteration per DPM | ||
| Max step trackind | 500 | ||
| Step length factor | 5 | ||
| Injection type: | Surface mass flow inlet | ||
| Diameter Distribution: | uniform | ||
| Diameter: | 0.0001 m | ||
| Total flow rate (kg/s) | 0.32 | ||
| Boundary Condition | |||
| Type | Amount (units) | ||
| Velocity inlet 1 | 0.184 m/s | ||
| Mass flow inlet 2 | 2.7 kg/s | ||
| pressure outlet (gauge pressure) | 0 pa | ||
| domain wall | |||
| wall | reflect | ||
| Cell zone condition | |||
| Fluid | Mixture-template | ||
| Turbulence models | |||
| K- | viscous model | ||
| SST | K- model | ||
| Solution methods | |||
| Coupled | pressure velocity coupling | ||
| Second-order | pressure | spatial discretization | |
| Second-order upwind | momentum | ||
| First-order upwind | turbulent kinetic energy | ||
| First-order upwind | turbulent dissipation rate | ||
| Second-order upwind | Energy | ||
Results
Observing the temperature contour inside the computational domain, it is clear that the average temperature inside the chamber is about 3200 Kelvin, and this temperature distribution with the boundary condition of argon gas’s mass flow rate of 2.7 kg/s and magnesium Oxide particle’s mass flow rate of 0.32 kg/s was obtained (Inlet 2). The maximum temperature inside the combustion chamber was monitored during the simulation process and reached a value of 3450 K, which indicates an increase in the chamber’s temperature due to the exothermic reaction.
In addition, in the mass fraction contours, it is clear that magnesium oxide decomposes during an exothermic reaction to magnesium and oxygen after entering the chamber.

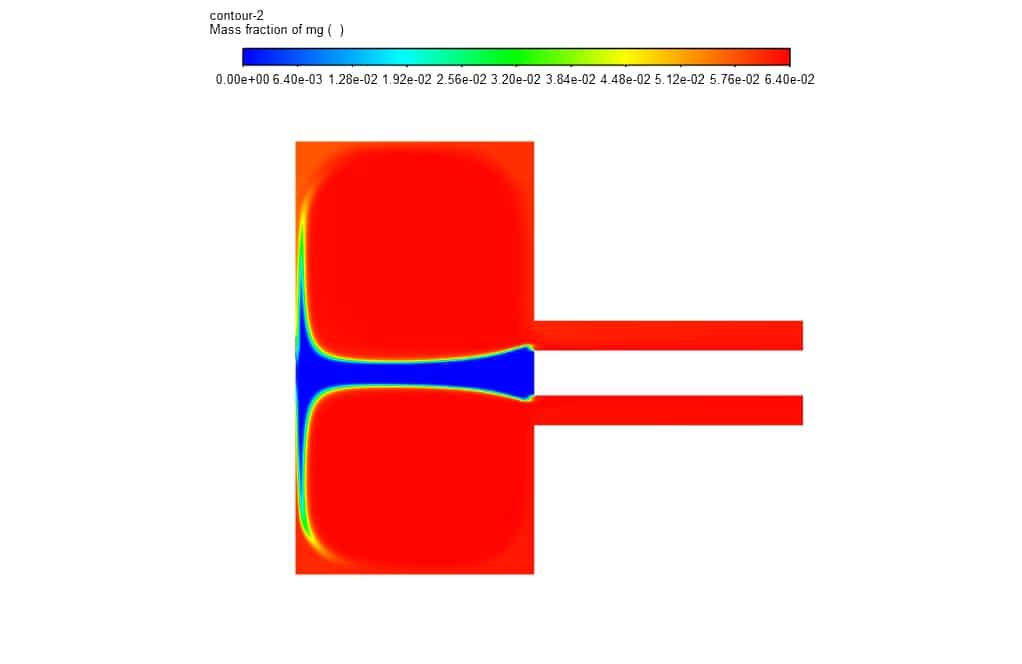
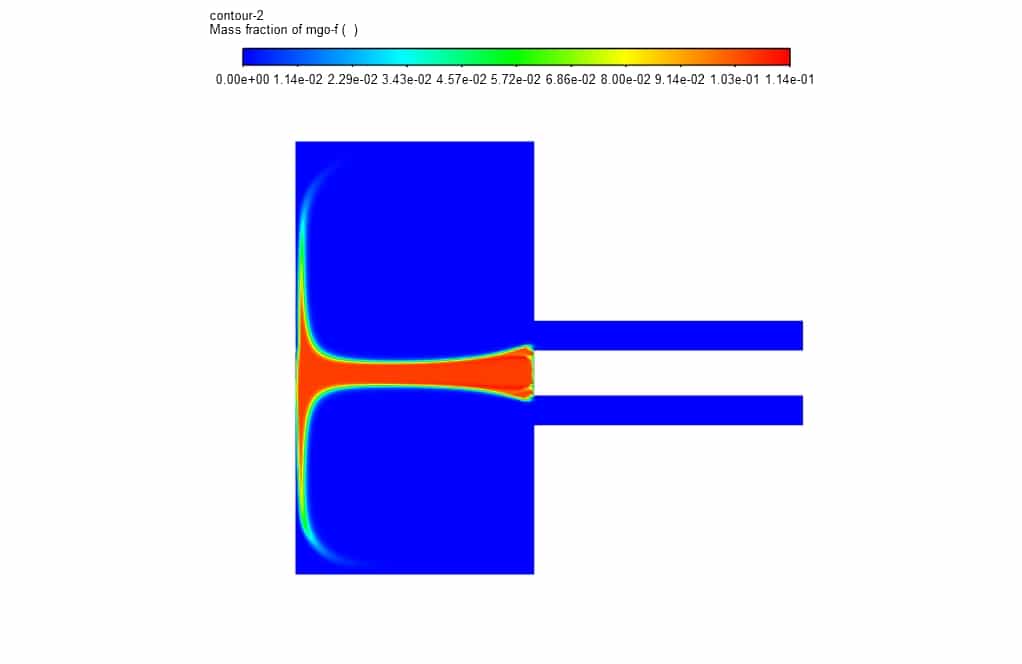
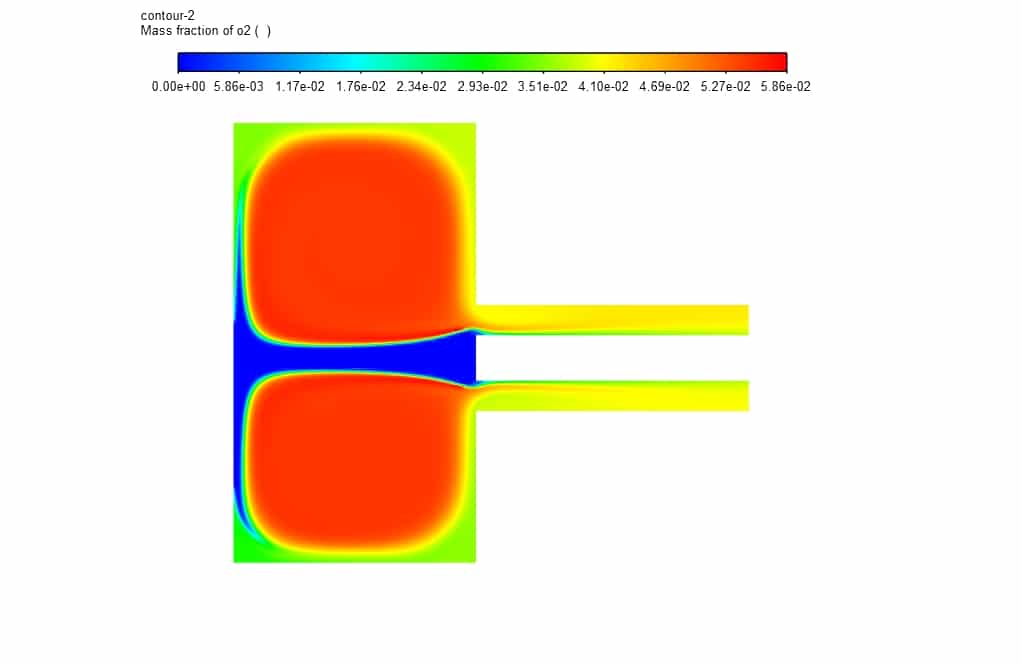
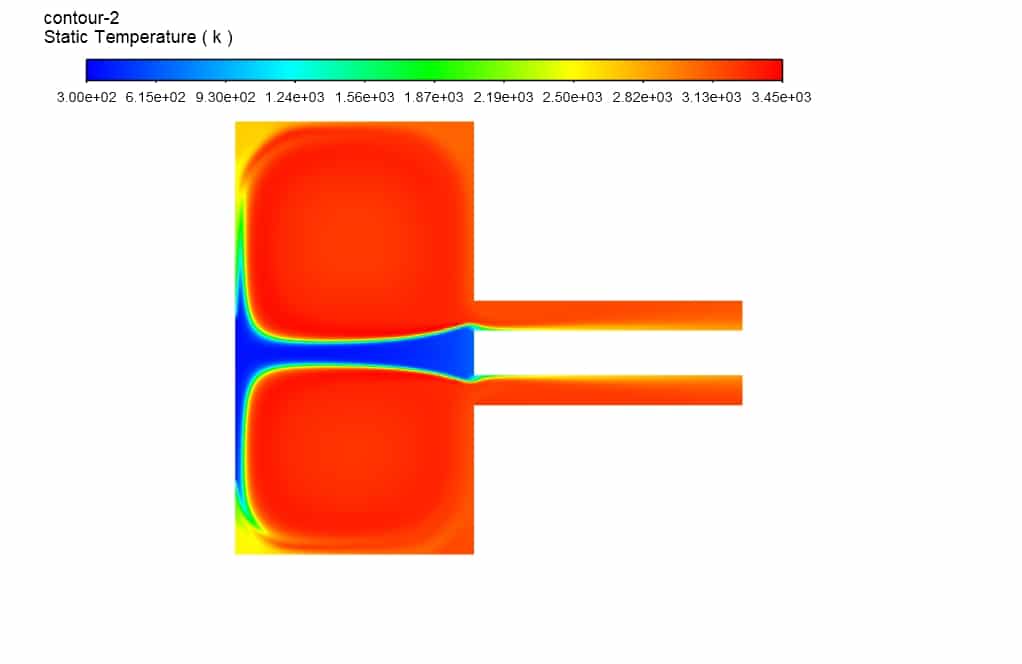



Kathryn Hackett –
The results show a significant rise in temperature in the combustion chamber. Does this imply that the intended thermolysis of magnesium oxide is effective in this simulation setup?
MR CFD Support –
The increase in temperature within the combustion chamber, reaching upwards of 3450 K, coupled with the mass fraction contours illustrating the decomposition of magnesium oxide into magnesium and oxygen, affirmatively indicates that the thermolysis process is effective under the presented simulation conditions. This successful simulation of magnesium oxide decomposition attests to the proficiency of ANSYS Fluent in accurately modeling thermal decompositions and achieving the desired chemical transformations.
Amaya Kuhlman –
The training content mentions the use of DPM and species transport methods. Could you provide more clarity on how DPM specifically contributes to modeling the decomposition of magnesium oxide in this simulation?
MR CFD Support –
In this training scenario, the Discrete Phase Model (DPM) contributes to the simulation by tracking the individual magnesium oxide particles injected into the domain. DPM calculates the particles’ trajectories, along with the heat, mass, and momentum exchange with the carrier phase, which is the argon gas. The species transport model then works in tandem with DPM to handle the chemical species involved and their interactions, including the decomposition reaction of magnesium oxide into magnesium and oxygen. This combination provides a detailed insight into decomposition kinetics and the particles’ behavior in the thermal environment.
Zelma Brown –
What controls the decomposition rate of magnesium oxide in this simulation, and can it be adjusted within the software?
MR CFD Support –
The decomposition rate of magnesium oxide is controlled primarily by the temperature settings, particle injection rates, and properties of argon gas used as a carrier and preheating medium. Furthermore, within the software, it’s possible to adjust parameters like inlet temperatures, flow rates of argon gas and magnesium oxide particles, and initial conditions to control the rate of decomposition.
Louvenia Cremin –
This training content on magnesium particle production using argon gas is exceptional! The step-by-step guide meticulously clarifies each phase of the process, from setting thermodynamic properties to interpreting the temperature and mass fraction contours. The attention to detail in explaining the decomposition reaction makes it a practical resource for learners like me who are looking to understand the complexities of CFD simulations in advanced material synthesis processes.
MR CFD Support –
We are overjoyed to hear that you found our training content insightful and practical! It’s always a pleasure to provide valuable resources to our learners and we take pride in sharing detailed information on such complex simulations. Thank you for your positive feedback, and we hope you continue to find our trainings beneficial for your endeavors.
Nikita Grant –
I’m curious, what diameter distribution for the magnesium oxide particles is typically considered when using a uniform diameter? Do the results vary significantly with particle size?
MR CFD Support –
In this study, the uniform diameter for the magnesium oxide particles set in the simulation is 0.0001 m. A uniform distribution means that each particle injected into the system has the same size. Results can vary with particle size because it influences the surface area available for reaction, and larger or smaller particles may have different reaction rates and heat transfer characteristics. The significant impact on reaction kinetics and dynamics due to particle size would need to be studied further through a sensitivity analysis to determine the exact extent of these effects.
Tom Quitzon –
I am thrilled with how detailed the decomposition process of magnesium oxide is observed through ANSYS Fluent. The results from the contours presenting the temperature and mass fraction are fascinating. It’s impressive seeing the exothermic reaction’s effect on the chamber temperature. Definitely clarified the theoretical aspects with practical insights!
MR CFD Support –
Thank you for your kind feedback! We at MR CFD are delighted to hear that our training course on the decomposition of magnesium oxide with argon gas was comprehensive and clear. It’s always rewarding to know that our course materials support our customers in both understanding the theory and observing its practical application in ANSYS Fluent. We appreciate your compliment!
Mrs. Roma Pollich –
I’m impressed with the clear temperature contours obtained. Could you tell me what specifically causes the high levels of temperature uniformity within the combustion chamber in this simulation?
MR CFD Support –
In the simulation, the temperature uniformity within the combustion chamber is mainly due to the optimized mass flow rates of argon and magnesium oxide particles. Additionally, the coupling of the DPM model with species transport ensures that the energy released by the exothermic reaction is well-distributed throughout the computational domain. The adequate mesh quality also plays a role in capturing temperature gradients accurately.
Mr. Timothy Kris DDS –
The description covers the process well. A job well done! I look forward to applying what I’ve learned.
MR CFD Support –
Thank you very much for your review! We’re delighted to hear that you found the training material informative and practical. It’s great to know that our resources are helping customers apply their knowledge effectively. If there’s anything else we can help you with, feel free to reach out. Happy learning!
Brendon Mueller –
I’ve always been interested in the chemistry behind thermal processes and your training on MgO decomposition with argon gas has been very enlightening! The detailed explanation on temperature contours and the exothermic reaction effects within the combustion chamber helped me understand the simulation workflow. Do you also address how different MgO particle sizes might affect the decomposition rate and efficiency in your simulations?
MR CFD Support –
We’re delighted to know that the training was enlightening for you! To directly address your question, while the training you’ve taken focuses on a specific particle size distribution and flow rates for MgO decomposition, the effects of different particle sizes on the rate and efficiency can certainly be explored. This would involve additional simulations varying the MgO particle size parameters within ANSYS Fluent’s Discrete Phase Model (DPM) framework to observe how it influences the overall kinetics and thermodynamics of the decomposition process. It’s an interesting area for further study, should anyone wish to deepen their understanding in particulate reactions.
Erick Simonis –
I was amazed by the results you achieved in the process! The thermal contours and the clarity of MgO decomposition within the simulation look impressive. It’s intriguing how you attain these accurate temperature distributions and particle behavior. Kudos to the MR CFD Team for providing such detailed training material for complex thermal analysis!
MR CFD Support –
We greatly appreciate your positive feedback! It’s wonderful to hear that you are satisfied with the detail and accuracy of our ANSYS Fluent Training for the MgO decomposition process. Our team strives to create comprehensive materials that facilitate a deep understanding of complex simulations. Should you require further exploration into CFD topics, rest assured that MR CFD will continue to provide high-quality resources. Thank you for choosing us for your learning journey!
Aliyah Parisian –
I successfully completed the ANSYS Fluent course on ‘Decomposition of MgO with Argon Gas for Magnesium Particle Production’ and I’m quite impressed with the deep dive into thermodynamics and simulation techniques used.
MR CFD Support –
Thank you for your kind words! We’re delighted to know the course met your expectations and provided you with valuable insights into the thermal decomposition process in CFD. If you have any further questions or need more training, please reach out to us.
Dayton Lubowitz –
I recently finished the ‘Decomposition of MgO with Argon Gas for Magnesium Particle Production’ course. I must say, the course materials are well-prepared and the simulation setup was thoroughly explained. However, could you please clarify if there was any model calibration involved? Specifically, was there a way to verify that the simulation results were accurate relative to experimental or theoretical results?
MR CFD Support –
Thank you for the positive feedback on the course material and the explanations provided. Regarding verification, yes, typically, simulation results are verified through comparison with experimental data or referencing theoretical results to ensure their accuracy. In this particular case, this would involve comparing the results obtained from the simulation regarding temperature distribution, magnesium, and oxygen production with literature data or experimental observations of MgO decomposition in argon gas. The specifics of whether such a calibration was explicitly conducted or available within this course would be detailed in the training materials or subsequent project documentation. If available, the calibration process helps improve the reliability of the simulation and provides better insight into the endothermic process parameters.
Kailyn Doyle PhD –
I’m interested in trying out the simulation! Could you tell me if pre-existing knowledge in thermodynamics and complex CFD is necessary, or can a beginner with basic CFD competence utilize this to learn and understand the process involved?
MR CFD Support –
No pre-existing knowledge in thermodynamics or complex CFD is needed. The simulation is designed to be user-friendly and offers detailed instructions to guide users through the process. Even individuals with just basic CFD understanding can benefit from and learn through this comprehensive training module. It covers all necessary concepts and techniques step by step.
Mrs. Alia Schamberger –
This training remarkably captures the intricacies of the decomposition process in magnesium manufacturing. The explanation of temperature variations, particularly the noteworthy increase to 3450 K in the reaction chamber, was both insightful and fascinating. The meticulous details on meshing, solver settings, and boundary conditions add huge value for any CFD enthusiast or professional.
MR CFD Support –
Thank you for your kind words! It’s fantastic to hear that the detail we’ve put into illustrating the decomposition process and the temperature variations has been insightful and of value to you. We strive to provide thorough explanations in our CFD training to enhance understanding and application. Don’t hesitate to reach out to us if you have any further questions or need more information on any topic!
Myrl Abshire –
The training was meticulous and seemed to cover all aspects. I just feel like hugging the entire MR CFD team. You folks are breaking grounds!
MR CFD Support –
Thank you for your heartfelt compliment! We’re thrilled to hear you had such a positive experience with our training. Your enthusiasm is truly appreciated, and it inspires us to keep delivering high-quality CFD education. If you ever have more feedback or need further assistance, we’re always here to help!
Emmanuel Russel –
The product was immensely helpful – I was particularly impressed with the clarification on how both the DPM and species transport methods were applied for analyzing the decomposition effects of magnesium oxide particles. The simulation details provided me with a strong understanding of the process.
MR CFD Support –
Thank you for your positive review. It is our goal to provide comprehensive learning materials that help demystify complex processes like the thermal decomposition of materials. We are pleased that the detail we put into explaining the use of DPM and species transport methods in this simulation was clear and beneficial for you. If you have any more questions or need further assistance, please do not hesitate to ask.
Julian Gibson –
The ANSYS Fluent training for magnesium particle production was terrific! The clarity of explanations and step-by-step procedures provided an in-depth understanding of the decomposition process, and I really appreciated the attention to detail in the material properties and boundary conditions. Wonderful work by the MR CFD team in setting up such intricate simulation parameters and providing insightful results.
MR CFD Support –
Thank you for your kind feedback! We’re delighted to hear that our training for the decomposition of MgO with Argon Gas in ANSYS Fluent met your expectations. Understanding every step of the simulation process is crucial to us, and we’re pleased it enhanced your learning experience. If you need further assistance or would like to explore more topics, feel free to reach out. Happy learning!
Ms. Lonie Lakin –
I noticed the inlet gases and particles have different temperatures. Can you explain how the preheated argon affects the decomposition process of magnesium oxide in the simulation?
MR CFD Support –
In the simulation set up by MR CFD, preheated argon gas is used to initiate the endothermic decomposition of magnesium oxide. The argon gas enters at two different temperatures, with the hotter stream including the magnesium oxide particles. The preheated argon provides the thermal energy required to overcome the activation energy barrier of the decomposition reaction. This heat transfer allows the magnesium oxide particles to decompose into magnesium and oxygen within the reactor chamber. The presence of the preheated argon helps to maintain a high temperature which is conducive to the overall chemical process.
Barrett Marvin –
The results show a very high chamber temperature resulting from the exothermic reaction. When injecting MgO at high temperatures with Argon as a carrier gas, how is the system ensured to be safe without any risk of unwanted reactions or safety hazards?
MR CFD Support –
To ensure the system is safe during operation, various safety measures are implemented. This includes monitoring the chamber temperature in real-time to prevent it from exceeding design limits. Physically, the materials used for constructing the chamber are chosen for their high melting points and structural stability at elevated temperatures. Additionally, appropriate emergency shutdown mechanisms are in place to counter any unsafe fluctuations immediately. Computational simulations like the one detailed here help predict and mitigate risks before real-world implementation.
Mona Collier –
The analysis sounds complex, but how is the generated oxygen managed within the simulation? Does it interact further with the magnesium particles or exit the chamber?
MR CFD Support –
In the simulation, oxygen as a product of the MgO decomposition reaction is tracked via the species transport equations. It doesn’t further interact with magnesium particles; instead, it gets carried by the argon gas flow and exits the chamber through the pressure outlet.
Helen Rohan MD –
The results mention an average temperature of 3200 Kelvin within the chamber. Could you clarify if this temperature is reached via the interaction of argon gas alone with MgO, or do other factors play a role in achieving this temperature?
MR CFD Support –
The average temperature of 3200 Kelvin within the chamber is primarily the result of the exothermic reaction of magnesium oxide decomposition into magnesium and oxygen. The preheated argon gas helps initiate this reaction, but the high temperature arises from the Exothermic energy released by the reaction itself, not from the interaction of argon gas alone with MgO
Prof. Hilton Koepp III –
Is the discrete phase model tracking also accounting for collisions with the chamber walls or just interactions with the continuous phase?
MR CFD Support –
In this study, the Discrete Phase Model (DPM) considers interactions with the continuous phase. It includes particle tracking through the continuous phase while accounting for forces acting on the particles. The model usually captures collision with the walls through reflection boundary conditions unless otherwise specified in the study details.
Harvey Feest –
I’m really intrigued by the way temperature distribution is visualized! What software or tools were used to create the temperature contour inside the computational domain?
MR CFD Support –
The temperature contour inside the computational domain was created using ANSYS Fluent software, which is capable of sophisticated simulation and visualization of thermal patterns in a reaction chamber as observed in this study.