Gas Flare, 2-step Air-Methane Mechanism Combustion, ANSYS Fluent Tutorial
$140.00 Student Discount
- The problem numerically simulates a two-step combustion mechanism in a gas flare using ANSYS Fluent software.
- We design the 3-D model by the Design Modeler software.
- We Mesh the model by ANSYS Meshing software, and the element number equals 1546925.
- We use the Species Transport model to define chemical species.
- The Volumetric option is selected to define chemical Reactions between reactants and products.
To Order Your Project or benefit from a CFD consultation, contact our experts via email ([email protected]), online support tab, or WhatsApp at +44 7443 197273.
There are some Free Products to check our service quality.
If you want the training video in another language instead of English, ask it via [email protected] after you buy the product.
Description
Description
The present problem simulates a two-step combustion mechanism in a gas flare in the presence of wind flow using ANSYS Fluent software. We perform this CFD project and investigate it by CFD analysis.
The flare system, also known as a gas flare, is a combustion device used in industrial units such as oil and gas refineries and the production of oil and gas wells, especially on offshore platforms.
The present model is designed in three dimensions using Design Modeler software. The present model is related to the construction of a gas flare due to symmetry, and only half of it has been modeled to reduce the computational cost.
This flare has a cylindrical structure with four outlet ducts located in a certain computational domain with the wind flow. This computational domain is also semi-designed due to symmetry, and the symmetry boundary condition is used.
The meshing of the model has been done using ANSYS Meshing software. The element number is 1,546,925.
Gas Flare Methodology
Gas flares are responsible for burning the natural gases released during oil extraction. In fact, during the oil extraction process, some natural gas accumulates in a mass on top of the oil in the reservoirs.
Therefore, collecting and storing natural gas is better, but they burn it if this is not possible. The combustion of these gases using the flare system causes, firstly, prevents the combustion and burning of these gases dangerously and uncontrollably.
Secondly, burning and converting methane to carbon dioxide and releasing them in the open space has less damage than the methane release.
Therefore, for this project, the species transport model has been used. The reaction mode must be activated in volumetric mode To define chemical reactions. The eddy dissipation model is also used to estimate the reaction rate.
In this simulation, a mixture of methane and air is burned in a two-step mechanism; they produce a chemical reaction in two consecutive stages.
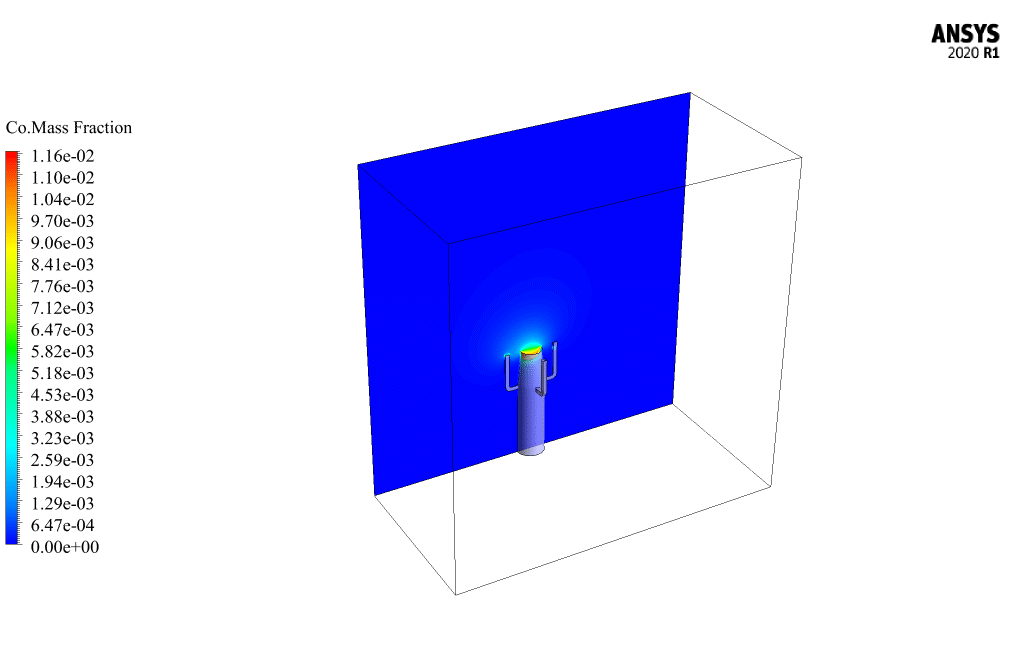
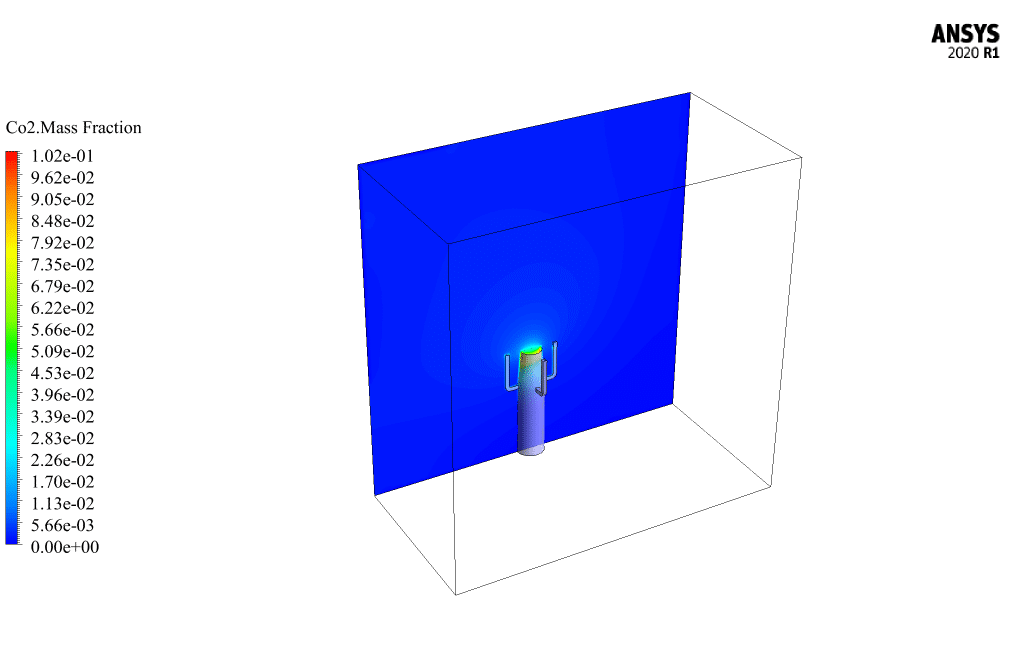
Initially, methane and oxygen react to produce carbon monoxide, and then carbon monoxide combines with oxygen to produce carbon dioxide. The air enters the domain with a velocity of 0.2 m/s and a temperature of 300K.
Also, the fuel enters with a velocity of 0.1 m/s and a temperature of 300K. Moreover, the realizable k-epsilon model and energy equation are enabled to solve the turbulent fluid equation and calculate temperature distribution within the domain.
Gas Flare Conclusion
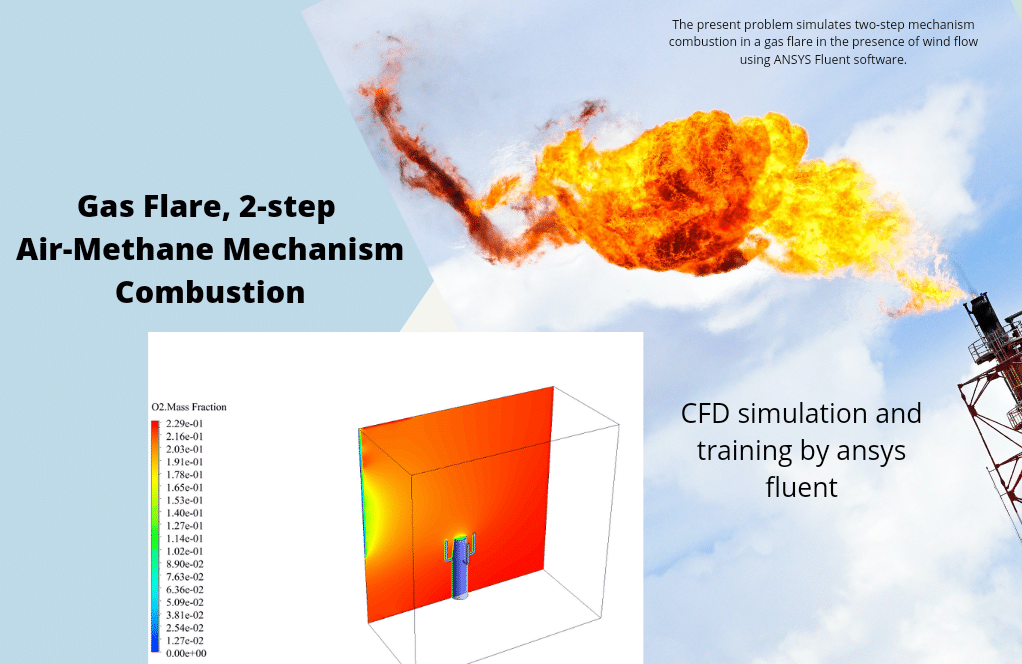
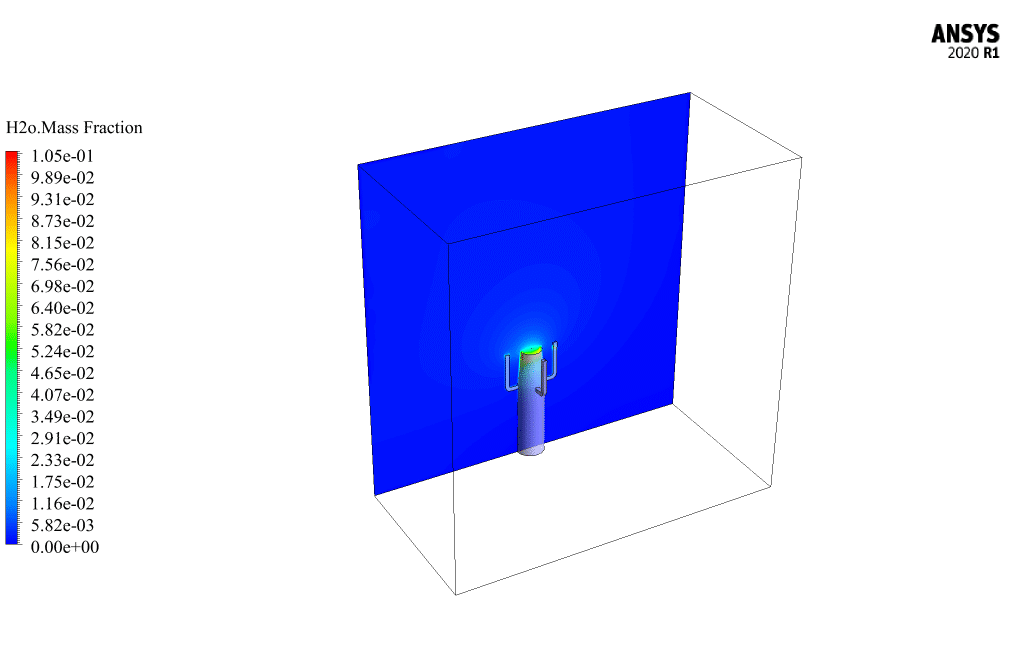
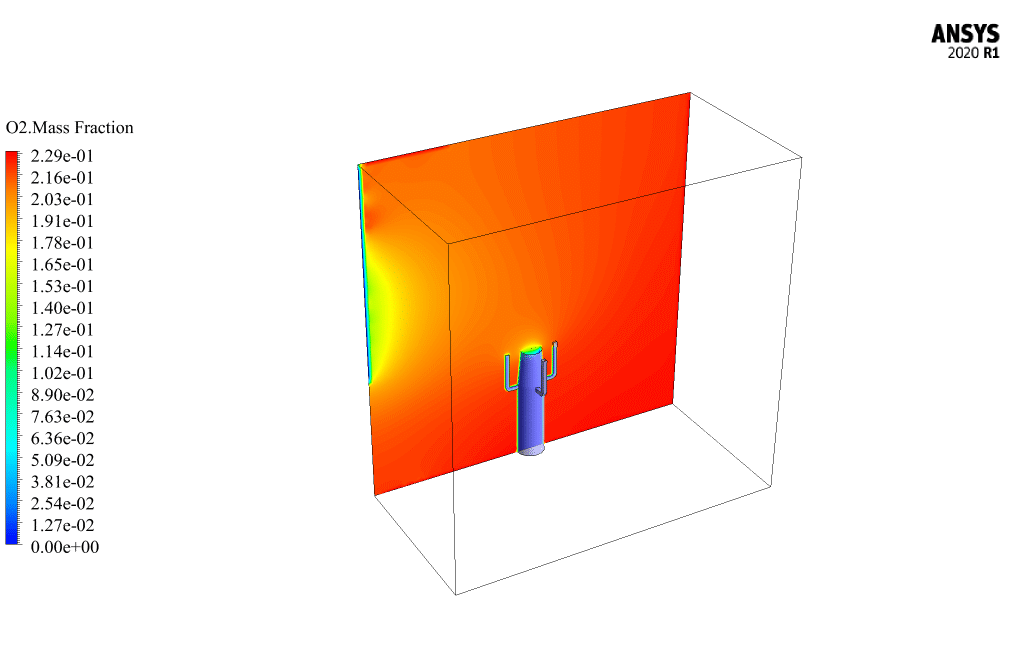
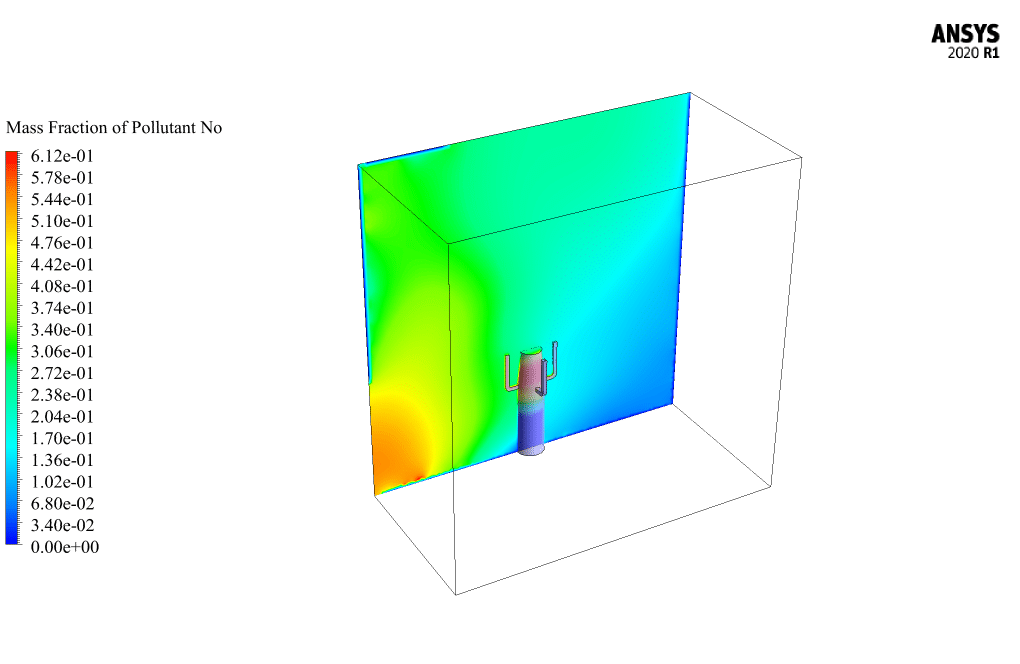
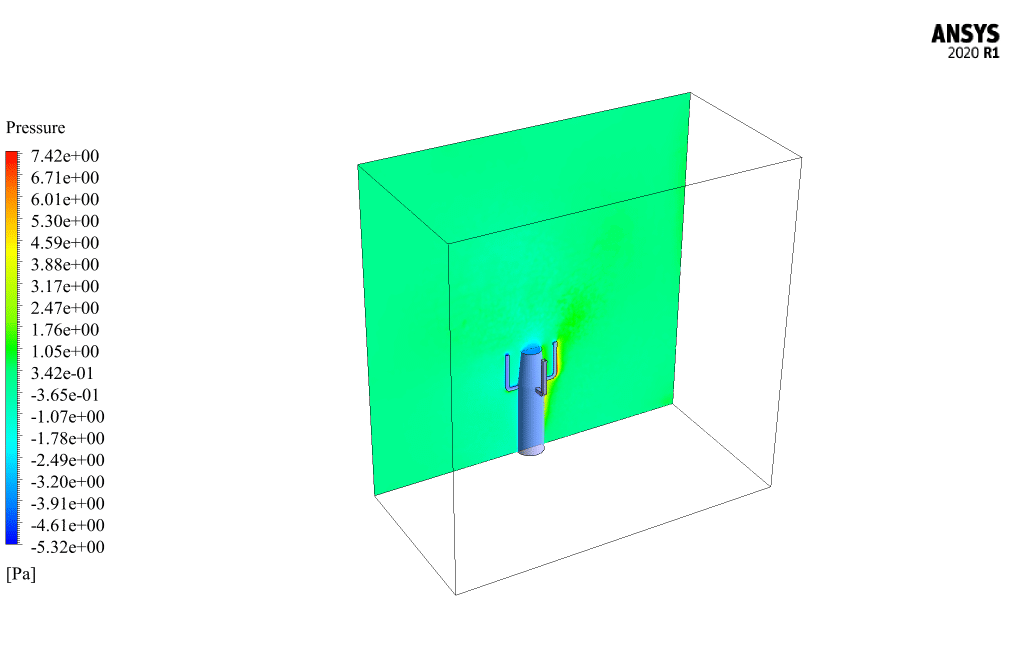
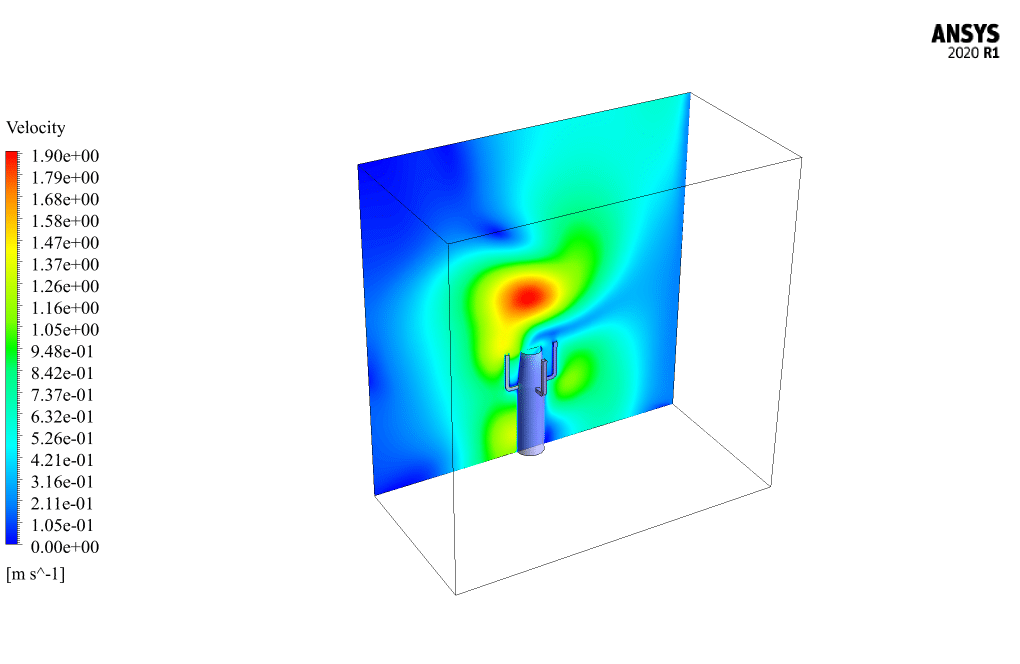
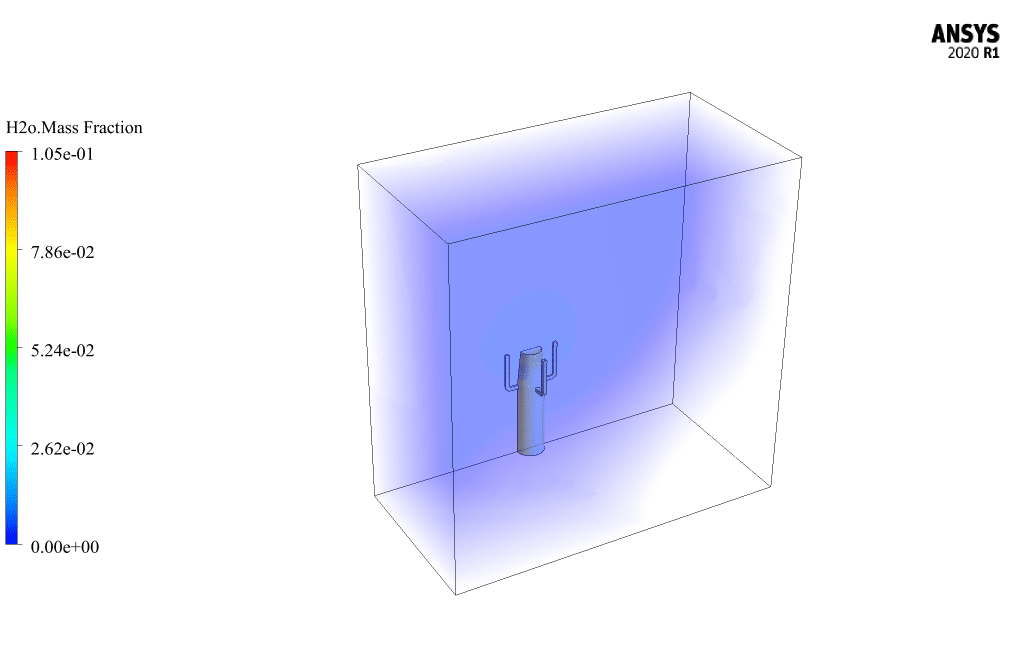
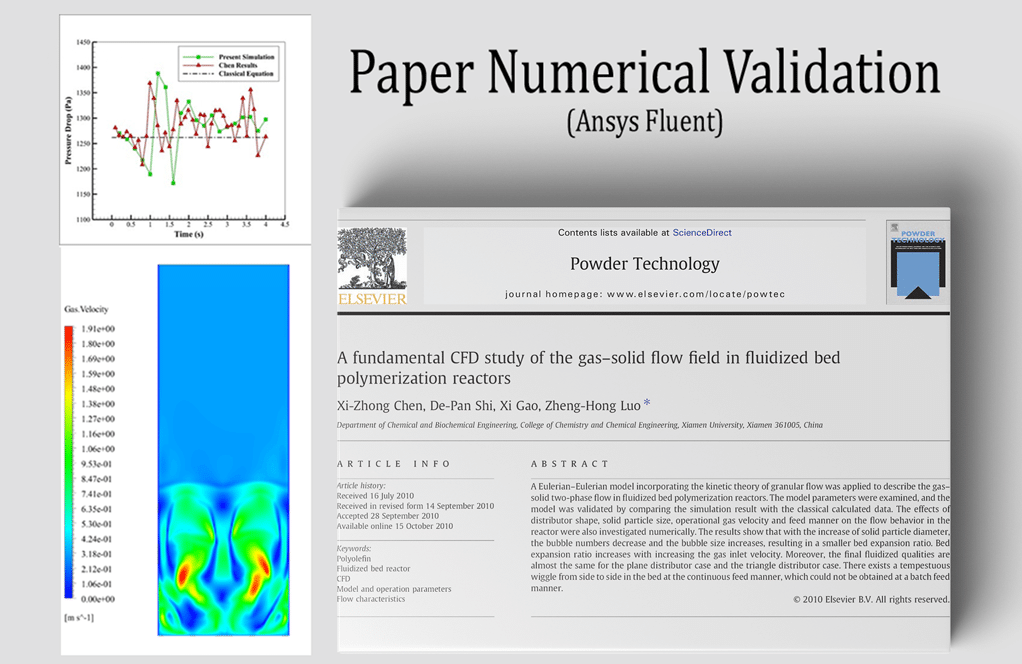
t the end of the solution process, two-dimensional and three-dimensional contours related to the pressure, temperature, velocity, and mass fraction of each gas species modeled in this simulation are obtained. Two-dimensional contours are displayed on the geometry symmetry plane.
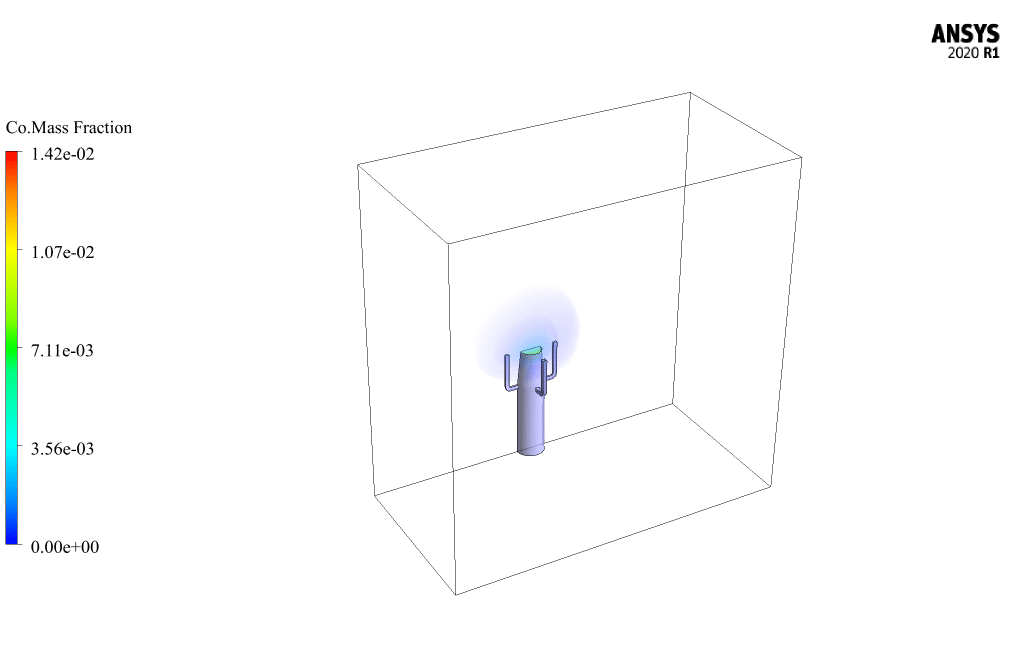
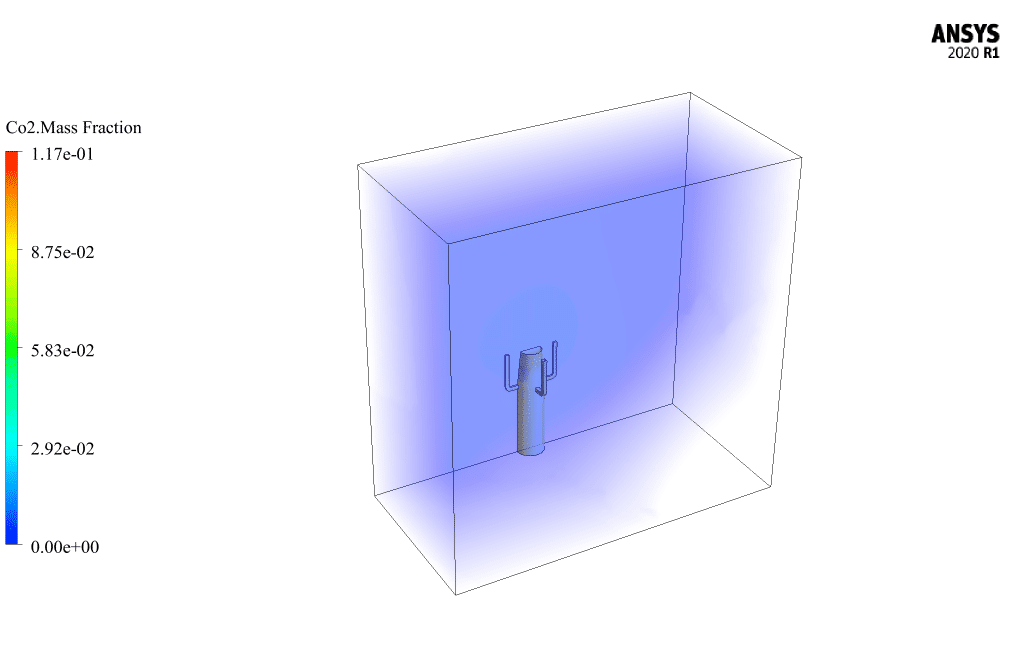
The mass fraction contour of gaseous species indicates the occurrence of a chemical reaction.
For example, the carbon dioxide and carbon monoxide contours indicate that a chemical reaction produces these gaseous products, or the methane contour indicates that the hydrocarbon is used as a chemical reaction agent.
Another noteworthy point is that the defined wind flow in the computational region causes the gases from the chemical reaction, such as carbon dioxide and carbon monoxide or NO pollutants, to be released into the environment around the flare.
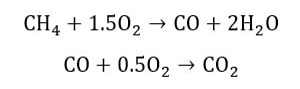

Theodora Schoen –
I just finished working through the Gas Flare, 2-step Air-Methane Mechanism Combustion tutorial by MR CFD, and wow – what a rush! The level of detail was incredible, ensuring that I could follow along with every step of the process without getting lost. Seeing the chemical reactions come to life on the screen was just mesmerizing. Now not only do I have a deeper understanding of combustion simulations, but I also feel equipped to tackle even more complex CFD problems in the future. Great job, MR CFD team – this tutorial is top-notch!
MR CFD Support –
Thank you so much for your amazing feedback! We’re absolutely thrilled to hear that you enjoyed the tutorial and found it highly effective for understanding the combustion process in a simulated gas flare. Knowing it has enhanced your confidence to approach more complex CFD challenges means a lot to us. Your satisfaction is our top priority, and messages like yours truly brighten our day. If you ever have more questions or need further assistance, remember we are just a message away. Keep up the great work!
Ms. Roberta Durgan –
I had such a great experience with the Gas Flare, 2-step Air-Methane Mechanism Combustion tutorial. The level of detail in explaining the combustion process and the reaction mechanism was impressive!
MR CFD Support –
Thank you for your kind words! We’re thrilled to hear that you found the tutorial informative and detailed. Our goal is to provide an in-depth understanding of the combustion process and it’s gratifying to know we’ve achieved this with you. If you have any more interest in our tutorials or additional questions, please feel free to reach out.
Addison Steuber –
This tutorial was immensely helpful for visualizing the combustion process! I was particularly impressed with the step-by-step explanations on setting up the species transport model and the chemical reactions.
MR CFD Support –
Thank you for your positive feedback! We are thrilled to hear that the tutorial was useful in helping you understand and visualize the combustion process using the species transport model and the details provided for the chemical reactions setup. It’s our goal to provide helpful and comprehensive guides for our users. If you have any further questions or need assistance with other projects, feel free to reach out. We’re here to help!
Lexi Jacobs –
The tutorial looks amazing! The detailed steps on methane and air combustion simulation have been described in an accessible manner, and I appreciate the visuals provided for the contours which made the complex process much more comprehensible!
MR CFD Support –
Thank you for your positive feedback on our ANSYS Fluent tutorial regarding gas flare combustion simulation. We’re thrilled to hear that you found the step-by-step instructions and visuals helpful and accessible. Your appreciation means a lot to us, and it motivates us to continue providing high-quality learning materials. If you have any further questions or need assistance with similar projects in the future, please don’t hesitate to reach out.
Ms. June Nicolas MD –
I’m thrilled with how well the two-step combustion mechanism has been presented. It’s impressive how the simulation captures the reaction stages producing carbon monoxide and then carbon dioxide. Well done!
MR CFD Support –
Thank you for your positive feedback. We’re delighted to hear that the simulation met your expectations and provided clear insight into the combustion process. If you have any further questions or need more information, feel free to reach out!
Olin Bosco –
The tutorial on gas flare simulation appears comprehensive. I was particularly impressed by how the two-step air-methane mechanism was explained. Could you share some insights on how the simulation’s realizable k-epsilon model impacts the evaluation of turbulence during this process?
MR CFD Support –
Thank you for your positive feedback! The realizable k-epsilon model in the simulation enhances the accuracy in predicting the spread and dissipation of turbulent kinetic energy. This model is robust and offers improved predictions for the airflow around the gas flare and the consequent turbulence that affects the flame characteristics and mixing of reactants in the combustion process.
Wellington Miller –
I was fascinated by the project’s method to reduce methane emissions through combustion. I want to learn more. Does this simulation include pollutant analysis like NOx formation during the combustion process?
MR CFD Support –
Yes, the simulation does include an analysis of the pollutants formed during the combustion process. By utilizing the species transport model and applying a two-step combustion mechanism, the formation of various gases, like carbon dioxide, carbon monoxide, and NOx (which includes various nitrogen oxide compounds), can be investigated. This way, the simulation provides insights into the environmental impact of gas flaring and helps in designing more efficient and less polluting flare systems.
Ms. Avis Glover II –
The simulation results are excellent. It is clear that a lot of work went into ensuring that the CFD project accurately portrays the combustion process and the effects of wind flow on gas flare. The use of a two-step mechanism is particularly impressive, as it provides a deeper understanding of the complexities around gas flares in industrial settings. I am pleased with how the contours illustrate the distribution of the different gaseous species. Great job!
MR CFD Support –
Thank you for your feedback and recognition of our efforts! We’re thrilled to hear that you’re satisfied with the simulation results and the level of detail provided by the two-step combustion mechanism. If there’s anything more we can help you with or information you’d like to discuss regarding CFD simulations, please let us know!
Jada Becker –
I was truly impressed by the way the simulation captivated the complex chemical reactions in a gas flare. The focus on the correct representation of chemical kinetics and the effects of wind on gas dispersion showcased top-notch engineering analysis. Fantastic job!
MR CFD Support –
Thank you for your positive feedback! We’re thrilled to hear that our simulation exceeded your expectations in representing the complexity of combustion and wind effects in gas flares. If you have any further queries or need additional information, feel free to ask. We’re always here to help.
Janet Wehner –
The tutorial was extremely helpful and detailed. The step-by-step approach made it a lot easier to follow along and actually understand the mechanisms behind gas flare combustion. The outcome of the simulation revealed intricate details about the chemical reactions which I found particularly insightful. Brilliant work MR CFD for such an in-depth tutorial!
MR CFD Support –
Thank you very much for your kind words! We are thrilled to hear that you found our tutorial on Gas Flare combustion with a 2-step air-methane mechanism to be helpful and insightful. We work hard to produce detailed and user-friendly educational content, so it’s very rewarding to see it appreciated. If you have any further questions or need assistance with other simulations, feel free to reach out. Thanks again for choosing MR CFD’s learning products!
Terrell Jacobs DVM –
Everything in this tutorial was carefully explained, making the complex combustion phenomena easier to understand. The simulation of the wind’s effect on gas flare discharge provided practical insights for real-world applications.
MR CFD Support –
Thank you so much for your positive feedback. We’re glad to hear that the tutorial made the understanding of complex combustion phenomena and the practical applications easier to grasp. If you have any further questions or need more information, we’re always here to help!
Celine Parker –
I was truly fascinated by how the gas flare CFD simulation showcases the complex combustion process. The focus on environmental aspects by detailing the conversion of methane to carbon dioxide to reduce the environmental impact was commendable.
MR CFD Support –
Thank you for your kind words! We’re pleased to know that our focus on environmental impact within the CFD simulation process resonated with you. Our team is committed to providing realistic simulations that not only achieve technical accuracy but also consider the broader implications on the environment. We appreciate your acknowledgment of our efforts!
Bernadine Jacobi –
I’ve watched through the Gas Flare CFD tutorial and the explanation of the combustion process is so clear. I can see there is also an emphasis on the environmental impact of flaring. Excellent insights for anyone learning about CFD applications in Environmental Engineering!
MR CFD Support –
Thank you for the kind words! We are glad that you found our tutorial on the simulation of two-step combustion mechanisms in a gas flare both informative and useful for understanding the environmental aspects of industrial processes. We are passionate about providing quality educational content that serves various learning purposes, and it’s great to know that it is making a difference. Your feedback is very encouraging for our team. If you have any further questions or need more insights on similar topics, don’t hesitate to reach out. Happy learning!
Verner Rogahn –
Beautifully detailed tutorial! The step-by-step progression made the complexities of a two-step combustion mechanism much more accessible. The intersection of theory and practical application was bridged smoothly, creating a learning experience that was both engaging and informative. Thank you for this thorough guide.
MR CFD Support –
We’re delighted to hear that you found our tutorial on the two-step combustion mechanism in a gas flare insightful. Thank you for taking the time to write such a positive review. It is our pleasure to provide resources that make complex CFD concepts easier to understand and apply. Your feedback is greatly appreciated!
Lyda Franecki –
Is the simulation considering the impact of wind on the combustion process and how does this influence the distribution of the gas species within the computational domain?
MR CFD Support –
Yes, the simulation accounts for the influence of wind on the gas flare’s combustion process. Wind flow is considered in the computational domain, causing the reactive gases to be affected by the wind’s direction and velocity. The wind impacts flame shape, mixing of the reactants, combustion efficiency, and the distribution of combustion products in the environment. In the post-processing of simulation results, you can observe how the evolved gases such as carbon dioxide and carbon monoxide are dispersed, which provides understanding of the environmental impact of the flare under the influence of wind.
Elvis Shields IV –
I was amazed at how well this simulation seemed to capture the real-world behaviors of gas mixing and combustion. The resulting methane contour clearly showed the consumption of hydrocarbons. Fabulous job on depicting this complex process in a clear, concise way!
MR CFD Support –
Thank you for your kind words! We are thrilled to hear that you found the simulation realistic and appreciated the clarity of the methane contour. Our team strives to deliver high-quality simulations that effectively represent complex physical phenomena. We are glad that our efforts resonate with users like you.
Mr. Candelario Jast –
Excellent tutorial! Your detailed explanation on how to simulate the gas flare’s two-step combustion mechanism made the process clear. Applying the species transport model along with the eddy dissipation model to handle the chemical reactions was well-founded, and showcasing the effects of wind on the combustion products was super insightful. Thanks for a first-rate educational resource!
MR CFD Support –
Thank you for your positive feedback! We are thrilled to hear that you found the tutorial clear and insightful. It’s great to know that the application of the simulation models was well-received and that you appreciated the detail on the wind effects. If you have any more questions or need further assistance, feel free to reach out to us. We’re always here to help you enhance your CFD skills.
Gage Lind –
I’m truly amazed by the realistic simulation of the gas flare combustion process. I appreciated how the transient behavior and the chemical complexities were incorporated into the CFD model, providing meaningful insights into emission patterns. Exemplary job on executing such a detailed analysis.
MR CFD Support –
Thank you for taking the time to share your positive experience. We are delighted to hear that you found the gas flare combustion simulation instructive and realistic. Your recognition of the complexity and detail of our analysis is much appreciated, and we take pride in providing thorough CFD solutions. Should you have any further inquiries or need additional information on our simulation projects, please feel free to reach out.